The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria - PubMed (original) (raw)
The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria
Sara C Di Rienzi et al. Elife. 2013.
Abstract
Cyanobacteria were responsible for the oxygenation of the ancient atmosphere; however, the evolution of this phylum is enigmatic, as relatives have not been characterized. Here we use whole genome reconstruction of human fecal and subsurface aquifer metagenomic samples to obtain complete genomes for members of a new candidate phylum sibling to Cyanobacteria, for which we propose the designation 'Melainabacteria'. Metabolic analysis suggests that the ancestors to both lineages were non-photosynthetic, anaerobic, motile, and obligately fermentative. Cyanobacterial light sensing may have been facilitated by regulators present in the ancestor of these lineages. The subsurface organism has the capacity for nitrogen fixation using a nitrogenase distinct from that in Cyanobacteria, suggesting nitrogen fixation evolved separately in the two lineages. We hypothesize that Cyanobacteria split from Melainabacteria prior or due to the acquisition of oxygenic photosynthesis. Melainabacteria remained in anoxic zones and differentiated by niche adaptation, including for symbiosis in the mammalian gut. DOI:http://dx.doi.org/10.7554/eLife.01102.001\.
Keywords: Cyanobacteria; Human; Melainabacteria; Other; human gut; nitrogen fixation; photosynthesis; subsurface.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
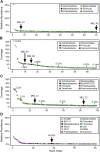
Figure 1.. Community composition of samples containing Melainabacteria.
(A–C) The relative composition of the human fecal samples A, B, and C, and (D) the aquifer community members. In A and D estimated percent relative abundance of the community is plotted, and in B and C, coverage is plotted, but estimated percent relative abundance is noted on the figure for select members. Organisms are classified at the phylum level. The human fecal sample A community is dominated by Prevotella copri DSM 18205, which accounts for more than 40% of the sequencing reads and is represented by several strains. Sequencing depth was not sufficient for human fecal sample C to accurately estimate roughly 25% of the community abundance, which includes MEL.C3. Aspects of the community composition of the aquifer sample are discussed in Wrighton et al. (2012). DOI:
http://dx.doi.org/10.7554/eLife.01102.004
Figure 2.. 16S rRNA gene phylogeny of Melainabacteria and Cyanobacteria.
Trees were built using 16S rRNA gene sequences from MEL.A1, MEL.B1, and MEL.B2 (the 16S rRNA sequence of ACD20 was not recovered). (A) 16S rRNA gene phylogeny tree with five representative sequences from each phylum obtained from the Greengenes May 2011 database (DeSantis et al., 2006). Bootstrap values greater than 50% are indicated. (B) 16S rRNA gene phylogeny built using one representative sequence from each order within Cyanobacteria from the Greengenes database (May 2011) (DeSantis et al., 2006) besides orders YS2, SM1D11, and mle1-12 from which all sequences were used. For Melainabacteria, the habitats from which the sequences were predominantly derived are indicated and colored according to isolation source (blue = environmental (non-gut); brown = gut). Cyanobacteria are displayed in green. Bootstrap values greater than 70% are indicated by a black square. DOI:
http://dx.doi.org/10.7554/eLife.01102.006
Figure 3.. Concatenated ribosomal protein phylogeny of the Melainabacteria and Cyanobacteria.
Maximum likelihood phylogeny and trait-based comparison of the eight novel organisms and 80 Cyanobacteria based on a concatenated protein alignment of 16 core ribosomal proteins from 733 taxa. In (A) the complete tree is shown at the phylum level and in (B) only the cyanobacterial-melainabacterial portion of the tree is shown. Bootstrap values >50% are indicated. Cyanobacteria branches are colored blue and Melainabacteria branches, red. The complete tree with all taxa shown is provided in Figure 3—figure supplement 1. The protein alignment on which this tree is based is provided in Figure 3—source data 1. DOI:
http://dx.doi.org/10.7554/eLife.01102.007
Figure 3—figure supplement 1.. Complete phylogeny of 733 taxa and the eight Melainabacteria based on a concatenated protein alignment of 16 core ribosomal proteins.
Melainabacteria branches are shown in red and Cyanobacteria branches in blue. Bootstrap values >50% are indicated. DOI:
http://dx.doi.org/10.7554/eLife.01102.009
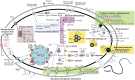
Figure 4.. The physiological and metabolic landscape of Melainabacteria.
Metabolic predictions for Melainabacteria based on genes identified in Figure 4—source data 1. Genes in pathways detected in the genomes of the subsurface and at least one gut genome (white box), only in the subsurface genome (grey box), only in at least one gut genome (orange box), and genes missing from pathways in all genomes (red box). Glycolysis proceeds via the canonical Embden-Meyerhof-Parnas (EMP) pathway with the exception of fructose-6-phosphate 1-phosphotransferase (EC:2.7.1.90, gene 3). Names of pathways and fermentation end-products are bolded and ATP generated by substrate-level phosphorylation are noted. All Melainabacteria genomes sampled lack electron transport chain components (including cytochromes (Cyto), succinate dehydrogenase (sdh), flavins, quinones), terminal respiratory oxidases or reductases, and photosystem I or II (PS1, PS2). The genomes also lack a complete TCA cycle (absent enzymes noted by red boxes), with the TCA enzymes instead linked to the fermentation of amino acids and organic acids denoted (pathways, blue arrows). Ferredoxin (Fd, green text) is important for hydrogen (H2) production via hydrogenases (yellow background box). Proton translocation mechanisms (green background box) may be achieved by the activity of trimeric oxaloacetate (OAA) decarboxylase and sodium-hydrogen antiporter, pyrophosphate (PPi) hydrolysis with pyrophosphatases, 11 subunit NADH dehydrogenase, and an annotated NiFe hydrogenase (green enzyme). Annotations for the gene numbers are in Figure 4—source data 2. The complete metabolic comparison of the Melainabacteria can be accessed at
http://ggkbase.berkeley.edu/genome\_summaries/81-MEL-Metabolic-Overview-June2013
. DOI:
http://dx.doi.org/10.7554/eLife.01102.010
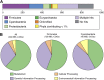
Figure 5.. MEL-COG phylum and functional assignments.
(A) Assignment of the 920 MEL-COGs (Figure 5—source data 1) to their best matching phyla. (B) Functional assignment of COGs by phylum assignment. Only COGs with functional assignments were considered. Number of MEL-COGs with no/multiple functional assignments are 532/42, 136/12, and 87/10 for All, Firmicutes, and Cyanobacteria, respectively. DOI:
http://dx.doi.org/10.7554/eLife.01102.013
Figure 5—figure supplement 1.. Distribution of MEL-COGs with best hits from different phyla across the MEL-A1 genome.
Gene distribution across the genome does not support large-scale recombination, lateral transfer events, or a chimeric genome assembly accounting for the presence of genes with greater similarity to genes from phyla other than Cyanobacteria. DOI:
http://dx.doi.org/10.7554/eLife.01102.015
Figure 6.. The phylogeny of the Melainabacteria nitrogenase.
A maximum likelihood phylogenetic tree constructed with 865 nitrogenase nifH genes from sequenced genomes (Zehr et al., 2003) is shown. nifH groups I and III are shown. The ACD20 nifH (in group III) is denoted in red, while photosynthetic cyanobacterial nifH sequences (in groups I and III) are denoted in green. Relative to group I, group III is characterized by deep bifurcations and long-branch lengths (Zehr et al., 2003), which are represented in the constructed tree by low-bootstrap values (<50) for internal branch positions in group III. ACD20 sequences are monophyletic (but with low bootstrap support) with nifH sequences from anaerobic Clostridium and Fusobacterium species. DOI:
http://dx.doi.org/10.7554/eLife.01102.016
Figure 7.. Putative TLR5 activation region in Melainabacteria flagellin genes.
Protein sequence alignment of residues 88–103 (Escherichia coli coordinates) for the flagellin genes. The range of residues required for TLR5 activation (Andersen-Nissen et al., 2005) are indicated by the top bracket. Sequences are organized by similarity within these residues. Species whose flagellin are reported (Andersen-Nissen et al., 2005) to be recognized (R) or unrecognized (UR) by TLR5 are noted. Based on the visualization of the alignment, flagellin genes predicted to be recognized or unrecognized by TLR5 are indicated; genes of ambiguous TLR5 recognition status are unmarked. DOI:
http://dx.doi.org/10.7554/eLife.01102.017
Figure 8.. Phylogeny of flagella-related genes.
Supertree (cladogram) of 13 bootstrap ML trees of the flagellar genes shared among the four analyzed genomes. The phylum (or more specific taxonomic identifier) of each species is listed: (F) Firmicutes, (S) Spirochaetes, (E-P) Epsilonproteobacteria, (MEL) Melainabacteria, (A) Aquificae, (T) Thermotogae, (D-P) Deltaproteobacteria, (Pl) Planctomycetes, (B) Bacteroidetes, (A-P) Alphaproteobacteria, (B-P) Betaproteobacteria, (G-P) Gammaproteobacteria. In all 13 individual trees, Melainabacteria branched with Firmicutes and Spirochaetes. DOI:
http://dx.doi.org/10.7554/eLife.01102.018
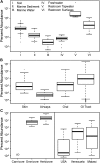
Figure 9.. The prevalence of members of Melainabacteria in different environments, including distinct human body habitats.
In all three panels, the relative abundances of Melainabacteria in different samples types are plotted as box plots (log10 transformed; i.e., 10−1 = 0.1%). Data were obtained from the QIIME database, derive from a variety of studies, and are publically available (Figure 9—source data 1): (A) soil, sediment, and water sites, (B) different human body sites (GI = gastrointestinal), (C, left) mammal stool classified by host diet, (C, right) country of origin for human stool. UD = undetermined. DOI:
http://dx.doi.org/10.7554/eLife.01102.019
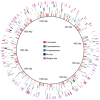
Figure 10.. Single copy gene inventory from reconstructed genomes.
Data are based on single copy genes (numbers in circles indicate the number of copies found). DOI:
http://dx.doi.org/10.7554/eLife.01102.021
Comment in
- Bacterial evolution: getting to the bottom of Cyanobacteria.
Hofer U. Hofer U. Nat Rev Microbiol. 2013 Dec;11(12):818-9. doi: 10.1038/nrmicro3158. Epub 2013 Oct 21. Nat Rev Microbiol. 2013. PMID: 24141638 No abstract available.
Similar articles
- An expanded genomic representation of the phylum cyanobacteria.
Soo RM, Skennerton CT, Sekiguchi Y, Imelfort M, Paech SJ, Dennis PG, Steen JA, Parks DH, Tyson GW, Hugenholtz P. Soo RM, et al. Genome Biol Evol. 2014 May;6(5):1031-45. doi: 10.1093/gbe/evu073. Genome Biol Evol. 2014. PMID: 24709563 Free PMC article. - Phylogenetic Diversity and Single-Cell Genome Analysis of "Melainabacteria", a Non-Photosynthetic Cyanobacterial Group, in the Termite Gut.
Utami YD, Kuwahara H, Murakami T, Morikawa T, Sugaya K, Kihara K, Yuki M, Lo N, Deevong P, Hasin S, Boonriam W, Inoue T, Yamada A, Ohkuma M, Hongoh Y. Utami YD, et al. Microbes Environ. 2018 Mar 29;33(1):50-57. doi: 10.1264/jsme2.ME17137. Epub 2018 Mar 8. Microbes Environ. 2018. PMID: 29415909 Free PMC article. - Phylogeny and Evolutionary History of Respiratory Complex I Proteins in Melainabacteria.
Grettenberger C, Sumner DY, Eisen JA, Jungblut AD, Mackey TJ. Grettenberger C, et al. Genes (Basel). 2021 Jun 18;12(6):929. doi: 10.3390/genes12060929. Genes (Basel). 2021. PMID: 34207155 Free PMC article. - Evolution of photosynthesis and aerobic respiration in the cyanobacteria.
Soo RM, Hemp J, Hugenholtz P. Soo RM, et al. Free Radic Biol Med. 2019 Aug 20;140:200-205. doi: 10.1016/j.freeradbiomed.2019.03.029. Epub 2019 Mar 29. Free Radic Biol Med. 2019. PMID: 30930297 Review. - Molecular aspects of nitrogen fixation by photosynthetic prokaryotes.
Hallenbeck PC. Hallenbeck PC. Crit Rev Microbiol. 1987;14(1):1-48. doi: 10.3109/10408418709104434. Crit Rev Microbiol. 1987. PMID: 3103981 Review.
Cited by
- Ecology and exploration of the rare biosphere.
Lynch MD, Neufeld JD. Lynch MD, et al. Nat Rev Microbiol. 2015 Apr;13(4):217-29. doi: 10.1038/nrmicro3400. Epub 2015 Mar 2. Nat Rev Microbiol. 2015. PMID: 25730701 Review. - Virtual 2D map of cyanobacterial proteomes.
Mohanta TK, Mohanta YK, Avula SK, Nongbet A, Al-Harrasi A. Mohanta TK, et al. PLoS One. 2022 Oct 3;17(10):e0275148. doi: 10.1371/journal.pone.0275148. eCollection 2022. PLoS One. 2022. PMID: 36190972 Free PMC article. - What's in a name? The case of cyanobacteria.
Garcia-Pichel F, Zehr JP, Bhattacharya D, Pakrasi HB. Garcia-Pichel F, et al. J Phycol. 2020 Feb;56(1):1-5. doi: 10.1111/jpy.12934. Epub 2019 Nov 15. J Phycol. 2020. PMID: 31618454 Free PMC article. - Astragalus and Ginseng Polysaccharides Improve Developmental, Intestinal Morphological, and Immune Functional Characters of Weaned Piglets.
Yang CM, Han QJ, Wang KL, Xu YL, Lan JH, Cao GT. Yang CM, et al. Front Physiol. 2019 Apr 12;10:418. doi: 10.3389/fphys.2019.00418. eCollection 2019. Front Physiol. 2019. PMID: 31031640 Free PMC article. - The Koala (Phascolarctos cinereus) faecal microbiome differs with diet in a wild population.
Brice KL, Trivedi P, Jeffries TC, Blyton MDJ, Mitchell C, Singh BK, Moore BD. Brice KL, et al. PeerJ. 2019 Apr 1;7:e6534. doi: 10.7717/peerj.6534. eCollection 2019. PeerJ. 2019. PMID: 30972242 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases