Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats - PubMed (original) (raw)
Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats
K Gradauer et al. J Control Release. 2013.
Abstract
The aim of the present study was the in vivo evaluation of thiomer-coated liposomes for an oral application of peptides. For this purpose, salmon calcitonin was chosen as a model drug and encapsulated within liposomes. Subsequently, the drug loaded liposomes were coated with either chitosan-thioglycolic acid (CS-TGA) or an S-protected version of the same polymer (CS-TGA-MNA), leading to an increase in the particle size of about 500 nm and an increase in the zeta potential from approximately -40 mV to a maximum value of about +44 mV, depending on the polymer. Coated liposomes were demonstrated to effectively penetrate the intestinal mucus layer where they came in close contact with the underlying epithelium. To investigate the permeation enhancing properties of the coated liposomes ex vivo, we monitored the transport of fluoresceinisothiocyanate-labeled salmon calcitonin (FITC-sCT) through rat small intestine. Liposomes coated with CS-TGA-MNA showed the highest effect, leading to a 3.8-fold increase in the uptake of FITC-sCT versus the buffer control. In vivo evaluation of the different formulations was carried out by the oral application of 40 μg of sCT per rat, either encapsulated within uncoated liposomes, CS-TGA-coated liposomes or CS-TGA-MNA-coated liposomes, or given as a solution serving as negative control. The blood calcium level was monitored over a time period of 24h. The highest reduction in the blood calcium level, to a minimum of 65% of the initial value after 6h, was achieved for CS-TGA-MNA-coated liposomes. Comparing the areas above curves (AAC) of the blood calcium levels, CS-TGA-MNA-coated liposomes led to an 8.2-fold increase compared to the free sCT solution if applied orally in the same concentration. According to these results, liposomes coated with S-protected thiomers have demonstrated to be highly valuable carriers for enhancing the oral bioavailability of salmon calcitonin.
Keywords: Chitosan; Liposome; Permeation enhancement; Salmon calcitonin; Thiomer.
© 2013. Published by Elsevier B.V. All rights reserved.
Figures
Graphical abstract
Fig. 1
Reaction of CS–TGA with 6,6′-dinicotinamide leads to the S-protected thiomer CS–TGA–MNA. Both polymers were used for the coating of preformed liposomes.
Fig. 2
Diffusion of rhodamine-labeled liposomes through porcine intestinal mucus. Mucus layers were incubated with uncoated liposomes (B), CS–TGA-coated liposomes (C), or CS–TGA–MNA coated liposomes (D) and compared with a control omitting any particles (A). Green (I) and red fluorescence (II) images were merged in order to distinguish between particles (red) and autofluorescence (yellow). The mucus layer thickness is displayed by the two dotted lines; the right side corresponds to the mucus surface, where particles have been applied.
Fig. 3
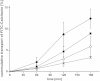
Absorptive permeation studies of FITC-calcitonin across rat intestinal tissue. Effect of uncoated liposomes (△) and liposomes coated with the thiolated chitosans CS–TGA (■) and the S-protected version CS–TGA–MNA (♦) in comparison to the FITC-sCT control (×). Indicated values are the means ± SD of at least three experiments.
Fig. 4
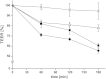
Decrease of the transepithelial electrical resistance (TEER) after adding uncoated liposomes (△) and liposomes coated with the thiolated chitosans CS–TGA (■) and its S-protected form CS–TGA–MNA (♦) in comparison to FITC-sCT control (×). Indicated values are the means ± SD of at least three experiments.
Fig. 5
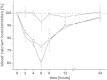
Effect of different application routes of sCT solution on the blood calcium level: The applied dose per rat was either 40 μg in case of an oral application (×), 2 μg in case of a s.c. application (□) or 1 μg for an i.v. injection (○). Indicated values are the means ± SD of three rats.
Fig. 6
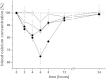
Decrease in the blood calcium level as a biological response to the oral application of sCT encapsulated within different liposomal formulations or given as a solution. sCT containing liposomes were coated with either CS–TGA (■) or CS–TGA–MNA (♦) and compared to uncoated, sCT loaded liposomes (△) and free sCT in solution (×). The applied dose per rat was 40 μg sCT in all cases. Indicated values are the means ± SD of three rats.
Similar articles
- Thiomer-coated liposomes harbor permeation enhancing and efflux pump inhibitory properties.
Gradauer K, Dünnhaupt S, Vonach C, Szöllösi H, Pali-Schöll I, Mangge H, Jensen-Jarolim E, Bernkop-Schnürch A, Prassl R. Gradauer K, et al. J Control Release. 2013 Feb 10;165(3):207-15. doi: 10.1016/j.jconrel.2012.12.001. Epub 2012 Dec 8. J Control Release. 2013. PMID: 23228848 Free PMC article. - Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties.
Gradauer K, Vonach C, Leitinger G, Kolb D, Fröhlich E, Roblegg E, Bernkop-Schnürch A, Prassl R. Gradauer K, et al. Int J Nanomedicine. 2012;7:2523-34. doi: 10.2147/IJN.S29980. Epub 2012 May 21. Int J Nanomedicine. 2012. PMID: 22679365 Free PMC article. - N-trimethyl chitosan-modified liposomes as carriers for oral delivery of salmon calcitonin.
Huang A, Makhlof A, Ping Q, Tozuka Y, Takeuchi H. Huang A, et al. Drug Deliv. 2011 Nov;18(8):562-9. doi: 10.3109/10717544.2011.596585. Epub 2011 Aug 8. Drug Deliv. 2011. PMID: 21823926 - [Absorption of calcitonin in oral and pulmonary administration with polymer-coated liposomes].
Takeuchi H, Sugihara H. Takeuchi H, et al. Yakugaku Zasshi. 2010 Sep;130(9):1135-42. doi: 10.1248/yakushi.130.1135. Yakugaku Zasshi. 2010. PMID: 20823671 Review. Japanese. - Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems.
Bernkop-Schnürch A, Kast CE, Guggi D. Bernkop-Schnürch A, et al. J Control Release. 2003 Dec 5;93(2):95-103. doi: 10.1016/j.jconrel.2003.05.001. J Control Release. 2003. PMID: 14636716 Review.
Cited by
- Micro/nanofabricated platforms for oral drug delivery.
Fox CB, Kim J, Le LV, Nemeth CL, Chirra HD, Desai TA. Fox CB, et al. J Control Release. 2015 Dec 10;219:431-444. doi: 10.1016/j.jconrel.2015.07.033. Epub 2015 Aug 2. J Control Release. 2015. PMID: 26244713 Free PMC article. Review. - Orally administered liposomal formulations for colon targeted drug delivery.
Hua S. Hua S. Front Pharmacol. 2014 Jun 10;5:138. doi: 10.3389/fphar.2014.00138. eCollection 2014. Front Pharmacol. 2014. PMID: 24959147 Free PMC article. No abstract available. - Adapting liposomes for oral drug delivery.
He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. He H, et al. Acta Pharm Sin B. 2019 Jan;9(1):36-48. doi: 10.1016/j.apsb.2018.06.005. Epub 2018 Jun 20. Acta Pharm Sin B. 2019. PMID: 30766776 Free PMC article. Review. - Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies-Reviewing Thiomers from Chitosan and Hyaluronic Acid.
Grosso R, de-Paz MV. Grosso R, et al. Pharmaceutics. 2021 Jun 8;13(6):854. doi: 10.3390/pharmaceutics13060854. Pharmaceutics. 2021. PMID: 34201403 Free PMC article. Review. - Thiolated Chitosan Conjugated Liposomes for Oral Delivery of Selenium Nanoparticles.
Selmani A, Seibert E, Tetyczka C, Kuehnelt D, Vidakovic I, Kornmueller K, Absenger-Novak M, Radatović B, Vinković Vrček I, Leitinger G, Fröhlich E, Bernkop-Schnürch A, Roblegg E, Prassl R. Selmani A, et al. Pharmaceutics. 2022 Apr 6;14(4):803. doi: 10.3390/pharmaceutics14040803. Pharmaceutics. 2022. PMID: 35456640 Free PMC article.
References
- IMARC . IMARC; 2013. Global Biopharmaceutical Market Report (2010–2015)
- Fricker G., Kromp T., Wendel A., Blume A., Zirkel J., Rebmann H., Setzer C., Quinkert R.O., Martin F., Muller-Goymann C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010;27:1469–1486. - PubMed
- Shukla D., Chakraborty S., Singh S., Mishra B. Lipid-based oral multiparticulate formulations — advantages, technological advances and industrial applications. Expert Opin. Drug Deliv. 2011;8:207–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources