Pds5B is required for cohesion establishment and Aurora B accumulation at centromeres - PubMed (original) (raw)
Pds5B is required for cohesion establishment and Aurora B accumulation at centromeres
María Carretero et al. EMBO J. 2013.
Abstract
Cohesin mediates sister chromatid cohesion and contributes to the organization of interphase chromatin through DNA looping. In vertebrate somatic cells, cohesin consists of Smc1, Smc3, Rad21, and either SA1 or SA2. Three additional factors Pds5, Wapl, and Sororin bind to cohesin and modulate its dynamic association with chromatin. There are two Pds5 proteins in vertebrates, Pds5A and Pds5B, but their functional specificity remains unclear. Here, we demonstrate that Pds5 proteins are essential for cohesion establishment by allowing Smc3 acetylation by the cohesin acetyl transferases (CoATs) Esco1/2 and binding of Sororin. While both proteins contribute to telomere and arm cohesion, Pds5B is specifically required for centromeric cohesion. Furthermore, reduced accumulation of Aurora B at the inner centromere region in cells lacking Pds5B impairs its error correction function, promoting chromosome mis-segregation and aneuploidy. Our work supports a model in which the composition and function of cohesin complexes differs between different chromosomal regions.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
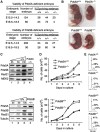
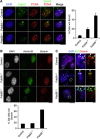
Effects of Pds5A or Pds5B ablation on mouse embryonic development and cell proliferation. (A) Viability of Pds5A- and Pds5B-deficient embryos. Heterozygous animals carrying a null allele of Pds5A (top) and Pds5B (bottom) were mated. (B) Pictures of littermate E18.5 embryos of the indicated genotypes. (C) Western blot analysis of whole-cell extracts prepared from mouse embryo fibroblasts (MEFs) obtained from E12.5 embryos homozygous for each knockout allele. The Pds5 antibodies recognize the most C-terminal region of Pds5A and Pds5B. Rad21 is a cohesin subunit. MEK2 or tubulin is used as a loading control. (D) Growth curves of primary MEFs of the indicated genotypes (two clones each). (E) Representative cell-cycle profiles obtained by FACS analysis of primary MEFs of the indicated genotypes. The numbers indicate the percentage of cells in G1, S, and G2/M according to the Dean-Jet-Fox model.
Figure 2
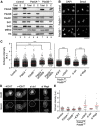
Levels of chromatin-bound cohesin in the absence of the Pds5 proteins. (A) Chromatin fractionation of MEFs wild type (control henceforth), Pds5A−/−, and Pds5B−/−. Equivalent amounts of total cell extract (Total), soluble fraction (S), and chromatin-enriched fraction (C) were loaded. Rad21, SA1, and SA2 are cohesin subunits. The cytoplasmic kinase MEK2 and histone H3 are used as controls for the fractionation procedure. (B) Representative images of primary MEFs of the indicated genotypes pre-extracted with detergent before fixation to remove soluble proteins and stained with Smc3. Scale bar, 50 μm. (C) Box plot showing the quantification of cohesin staining (Smc1 or Smc3, in arbitrary units and normalized to the average value obtained in control cells) in interphase cells of the indicated genotypes and conditions. Western blot analyses to assess the efficiency of siRNA treatments are shown in Supplementary Figure S3. The following numbers of cells (n) from several clones (N) were measured: control, _n_=972, _N_=7; Pds5A−/− _n_=698, _N_=4; Pds5B−/−, _n_=742 cells, _N_=6; Pds5A−/− siPds5B, _n_=334, _N_=2; Pds5B−/− siPds5A, _n_=482, _N_=5; Pds5Af/f;Pds5Bf/f;Cre-ERT2 MEFs −4OHT and +4OHT, _n_=700 each, _N_=1; control and Wapl siRNA, _n_=600 each, _N_=1. Bonferroni’s multiple comparison test was used to assess significance. ***P<0.001. (D) Examples of primary MEFs in metaphase (surrounded by dotted line) treated with detergent before fixation and stained with Smc3. (E) Cohesin staining was measured in at least 35 metaphases for the indicated genotypes or condition except for control and si ctr and si Wapl in which _n_=19 and 26, respectively. Scale bar, 10 μm.
Figure 3
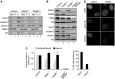
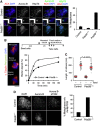
Pds5A and Pds5B are required for full acetylation of cohesin and Sororin recruitment. (A) Western blot analysis of the chromatin fractionation shown in Figure 2A with additional antibodies. (B) Western blot analysis of the chromatin fraction of primary MEFs of the indicated genotypes untransfected or transfected with siRNAs against Pds5A (siPds5A). (C) Quantification of the indicated signals from the two experiments shown above and an additional one not shown. Bars represent mean+s.e.m. (D) G2 cells in asynchronous cultures of Pds5Af/f;Pds5Bf/f;Cre-ERT2 MEFs grown in the absence or presence of 4-OHT were stained with Aurora B and Sororin antibodies and counterstained with DAPI. The percentage of cells labelled by both Aurora B and Sororin was quantified among >160 G2 cells from two different clones and plotted in the graph below the images. Scale bar, 10 μm.
Figure 4
Different requirements for Pds5A and Pds5B in telomere and centromere cohesion. (A) Metaphase chromosomes were stained with a telomeric repeat probe (red) and DAPI (blue). The image on the left corresponds to a wild-type cell. The fraction of fragile telomeres, that is, not round but elongated or double signals (red arrowhead) was measured for the indicated number of chromosomes from six clones control MEFs, three clones of Pds5A null or Pds5B null MEFs, two clones of SA1 null MEFs and one clone of the rest, and plotted (right). +4OHT refers to Pds5Af/f;Pds5Bf/f;Cre-ERT2 MEFs treated with 4-OHT for 5 days. (B) Quantification of breaks along the chromosome arms in metaphase chromosomes from cells either untreated (grey bars) or treated with 0.5 μM aphidicolin for 24 h (black bars). The images on the left show examples of the broken chromosomes (red arrowheads) in a Pds5B−/− cell. We counted the indicated number of chromosomes from two clones in all cases except Pds5B−/−siPds5A in which a single clone was used. (C) Representative images of metaphase spreads from control (left) and Pds5B−/− MEFs (right) showing proper and defective centromere cohesion, respectively. The graph below represents the percentage of cells showing more than five chromosomes with centromere cohesion defects among _n_⩾100 metaphase cells of each condition. We did not count metaphases showing only single chromatids, that is, in which pairs of sisters cannot be recognized. Scale bar, 20 μm.
Figure 5
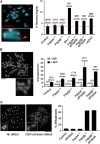
Esco2 acetylation of cohesin and Sororin recruitment at PCH depend on Pds5B. (A) Esco2 (green) accumulates at the late replicating heterochromatin, labelled by PCNA (red), of control and Pds5A−/− MEFs, but not in Pds5B−/− MEFs. For the graph on the right, we counted the fraction of Esco2-negative/PCNA horseshoe-positive cells among cells (n) from several clones (N): control, _n_=168, _N_=2; Pds5A−/−, _n_=57; _N_=3; Pds5B−/− _n_=177, _N_=3. Scale bar, 10 μm. (B) G2 cells labelled by Aurora B (green) showing reduced accumulation of Sororin (red) at DAPI-dense foci of pericentric heterochromatin were scored among 300, 506, and 713 G2 cells in 2 clones each of control, Pds5A−/−, and Pds5B−/− MEFs. Scale bar, 10 μm. (C) Metaphase chromosomes prepared by cytospin were labelled with Sororin (red), ACA (anti-centromere antibody, green), and DAPI (blue). While Sororin stains the inner centromere region of control and Pds5A null chromosomes, it is absent from chromosomes lacking Pds5B. Scale bar, 10 μm; inset, 1 μm.
Figure 6
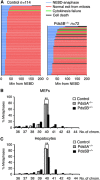
Chromosome segregation defects and aneuploidy in the absence of Pds5B. (A) Graphical summary of the progression through mitosis of control (_n_=114) and Pds5B−/− MEFs (_n_=72), as observed by live-cell imaging after transfection with H2B-mCherry (red) to label chromatin. Each line represents the progression through mitosis of a single cell and it is coloured according to the legend shown above the graph. Nuclear envelope breakdown (NEBD) is considered as _t_=0. Still images from some of the movies can be found in Supplementary Figure S6. (B) Graph showing the distribution in the number of chromosomes of at least 80 metaphases from 4 clones of control, 2 clones Pds5A−/− and 5 clones of Pds5B−/− primary MEFs. **P<0.005 (unpaired _t_-test). (C) Same analysis in cells from hepatocytes taken from E14.5 embryos. We examined 165 metaphases from three wild-type embryos, 165 metaphases from 2 Pds5A null embryos, and 109 metaphases from 4 Pds5B null embryos. **P<0.005 (unpaired _t_-test).
Figure 7
Pds5B is required for proper localization and activation of Aurora B. (A) Metaphase chromosomes prepared by cytospin were labelled with Aurora B, H3pT3, ACA, and DAPI. In control cells, and also in Pds5A null cells, Aurora B accumulates between the sister kinetochores labelled by ACA (top). In Pds5B−/− cells, Aurora B and H3pT3 signals appear all along chromosome arms and their centromeric enrichment is dramatically reduced (bottom). Scale bar, 10 μm. For the graph on the right, at least 85 metaphases from four different clones were scored for each genotype. Data represent mean+s.e.m. (B) Experimental design for the monastrol washout experiment (top) and representative images of the spindles observed after staining with alpha tubulin (red), pericentrin (green), and DAPI (blue). Between 65 and 175 spindles were counted in each time point for each genotype. (C) Box plot showing the duration of mitosis (as measured by live-cell imaging from NEBD to chromosome decondensation) in control and Pds5B−/− MEFs treated or not with taxol. Thirty-six cells were scored for each condition. (D) Metaphase chromosomes from control and Pds5B−/− MEFs prepared by cytospin were labelled with Aurora B and phospho(T232)Aurora B. We scored the staining of 58 metaphases from 2 control clones and 45 metaphases from 2 Pds5B−/− clones. Scale bar, 10 μm.
Figure 8
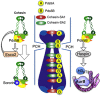
Specific cohesin complexes function at different chromosomal regions. A schematic representation of the specificity of different cohesin complexes (with SA1 or SA2 and Pds5A or Pds5B) to perform cohesion in different regions of the chromosome. Whether this functional distribution reflects a specific localization of the corresponding complexes is not known. The presence of Pds5B at pericentric heterochromatin (PCH) promotes cohesin acetylation by Esco2 during DNA replication and Sororin recruitment. Pds5B is also important for proper localization of Aurora B at the inner centromere region of mitotic chromosomes, maybe by promoting Haspin recruitment in mitosis.
Similar articles
- A kinase-dependent role for Haspin in antagonizing Wapl and protecting mitotic centromere cohesion.
Liang C, Chen Q, Yi Q, Zhang M, Yan H, Zhang B, Zhou L, Zhang Z, Qi F, Ye S, Wang F. Liang C, et al. EMBO Rep. 2018 Jan;19(1):43-56. doi: 10.15252/embr.201744737. Epub 2017 Nov 14. EMBO Rep. 2018. PMID: 29138236 Free PMC article. - The N-Terminal Non-Kinase-Domain-Mediated Binding of Haspin to Pds5B Protects Centromeric Cohesion in Mitosis.
Zhou L, Liang C, Chen Q, Zhang Z, Zhang B, Yan H, Qi F, Zhang M, Yi Q, Guan Y, Xiang X, Zhang X, Ye S, Wang F. Zhou L, et al. Curr Biol. 2017 Apr 3;27(7):992-1004. doi: 10.1016/j.cub.2017.02.019. Epub 2017 Mar 23. Curr Biol. 2017. PMID: 28343965 - The specific contributions of cohesin-SA1 to cohesion and gene expression: implications for cancer and development.
Cuadrado A, Remeseiro S, Gómez-López G, Pisano DG, Losada A. Cuadrado A, et al. Cell Cycle. 2012 Jun 15;11(12):2233-8. doi: 10.4161/cc.20318. Epub 2012 Jun 15. Cell Cycle. 2012. PMID: 22617390 - Releasing the cohesin ring: A rigid scaffold model for opening the DNA exit gate by Pds5 and Wapl.
Ouyang Z, Yu H. Ouyang Z, et al. Bioessays. 2017 Apr;39(4). doi: 10.1002/bies.201600207. Epub 2017 Feb 21. Bioessays. 2017. PMID: 28220956 Review. - The expanding phenotypes of cohesinopathies: one ring to rule them all!
Piché J, Van Vliet PP, Pucéat M, Andelfinger G. Piché J, et al. Cell Cycle. 2019 Nov;18(21):2828-2848. doi: 10.1080/15384101.2019.1658476. Epub 2019 Sep 13. Cell Cycle. 2019. PMID: 31516082 Free PMC article. Review.
Cited by
- Repression of Transcription at DNA Breaks Requires Cohesin throughout Interphase and Prevents Genome Instability.
Meisenberg C, Pinder SI, Hopkins SR, Wooller SK, Benstead-Hume G, Pearl FMG, Jeggo PA, Downs JA. Meisenberg C, et al. Mol Cell. 2019 Jan 17;73(2):212-223.e7. doi: 10.1016/j.molcel.2018.11.001. Epub 2018 Dec 13. Mol Cell. 2019. PMID: 30554942 Free PMC article. - Brca2, Pds5 and Wapl differentially control cohesin chromosome association and function.
Misulovin Z, Pherson M, Gause M, Dorsett D. Misulovin Z, et al. PLoS Genet. 2018 Feb 15;14(2):e1007225. doi: 10.1371/journal.pgen.1007225. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29447171 Free PMC article. - The cohesion stabilizer sororin favors DNA repair and chromosome segregation during mouse oocyte meiosis.
Huang CJ, Yuan YF, Wu D, Khan FA, Jiao XF, Huo LJ. Huang CJ, et al. In Vitro Cell Dev Biol Anim. 2017 Mar;53(3):258-264. doi: 10.1007/s11626-016-0107-0. Epub 2016 Nov 8. In Vitro Cell Dev Biol Anim. 2017. PMID: 27826797 - Embryonic Lethality in Homozygous Human Her-2 Transgenic Mice Due to Disruption of the Pds5b Gene.
Yong CS, Sharkey J, Duscio B, Venville B, Wei WZ, Jones RF, Slaney CY, Mir Arnau G, Papenfuss AT, Schröder J, Darcy PK, Kershaw MH. Yong CS, et al. PLoS One. 2015 Sep 3;10(9):e0136817. doi: 10.1371/journal.pone.0136817. eCollection 2015. PLoS One. 2015. PMID: 26334628 Free PMC article. - Disruption of NIPBL/Scc2 in Cornelia de Lange Syndrome provokes cohesin genome-wide redistribution with an impact in the transcriptome.
Garcia P, Fernandez-Hernandez R, Cuadrado A, Coca I, Gomez A, Maqueda M, Latorre-Pellicer A, Puisac B, Ramos FJ, Sandoval J, Esteller M, Mosquera JL, Rodriguez J, Pié J, Losada A, Queralt E. Garcia P, et al. Nat Commun. 2021 Jul 27;12(1):4551. doi: 10.1038/s41467-021-24808-z. Nat Commun. 2021. PMID: 34315879 Free PMC article.
References
- Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C, Hieter P (2008) Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci USA 105: 3443–3448 - PMC - PubMed
- Chan KL, Palmai-Pallag T, Ying S, Hickson ID (2009) Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat Cell Biol 11: 753–760 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous