Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function - PubMed (original) (raw)
Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function
Amy J Eshleman et al. Psychopharmacology (Berl). 2014 Mar.
Abstract
Rationale: Psychoactive-substituted phenethylamines 2,5-dimethoxy-4-chlorophenethylamine (2C-C); 2,5-dimethoxy-4-methylphenethylamine (2C-D); 2,5-dimethoxy-4-ethylphenethylamine (2C-E); 2,5-dimethoxy-4-iodophenethylamine (2C-I); 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2); and 2,5-dimethoxy-4-chloroamphetamine (DOC) are used recreationally and may have deleterious side effects.
Objectives: This study compares the behavioral effects and the mechanisms of action of these substituted phenethylamines with those of hallucinogens and a stimulant.
Methods: The effects of these compounds on mouse locomotor activity and in rats trained to discriminate dimethyltryptamine, (-)-DOM, (+)-LSD, (±)-MDMA, and S(+)-methamphetamine were assessed. Binding and functional activity of the phenethylamines at 5-HT1A, 5-HT2A, 5-HT2C receptors and monoamine transporters were assessed using cells heterologously expressing these proteins.
Results: The phenethylamines depressed mouse locomotor activity, although 2C-D and 2C-E stimulated activity at low doses. The phenethylamines except 2C-T-2 fully substituted for at least one hallucinogenic training compound, but none fully substituted for (+)-methamphetamine. At 5-HT1A receptors, only 2C-T-2 and 2C-I were partial-to-full very low potency agonists. In 5-HT2A arachidonic acid release assays, the phenethylamines were partial to full agonists except 2C-I which was an antagonist. All compounds were full agonists at 5-HT2A and 5-HT2C receptor inositol phosphate assays. Only 2C-I had moderate affinity for, and very low potency at, the serotonin transporter.
Conclusions: The discriminative stimulus effects of 2C-C, 2C-D, 2C-E, 2C-I, and DOC were similar to those of several hallucinogens, but not methamphetamine. Additionally, the substituted phenethylamines were full agonists at 5-HT2A and 5-HT2C receptors, but for 2C-T-2, this was not sufficient to produce hallucinogen-like discriminative stimulus effects. Additionally, the 5-HT2A inositol phosphate pathway may be important in 2C-I's psychoactive properties.
Conflict of interest statement
Conflict of interest.
All authors declare that they have no conflicts of interest.
Figures
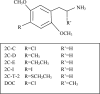
Fig. 1
Structures of 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2 and DOC
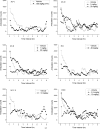
Fig. 2
Average horizontal activity counts/10 min (ambulation counts) as a function of time (0–8 hr) and dose of test compound. Data for the vehicle and the dose which produced peak depressant effects are shown in each panel. 2C-D and DOC also produced stimulant effects, and data for the dose which produced peak stimulant effects are also shown. N=8 for each treatment.
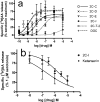
Fig. 3
[3H]AA release from HEK-5-HT2A cells. Experiments were conducted as described in methods. Data presented are means ± sem. (A) Agonist assay. Basal activity is subtracted, and data are normalized to the maximal stimulation by serotonin on each experimental day. n=3–5 except n=2 for 2C-I. (B) Antagonist assay. Nonspecific release, measured in the presence of 30 μM ketanserin, is subtracted from all data and data are normalized to the maximal release stimulated by serotonin. n=3–4.
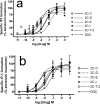
Fig. 4
Stimulation of IP-1 formation in HEK-5-HT2A and HEK-5-HT2C cells. Experiments were conducted as described in methods. A. HEK-5-HT2A cells. All compounds are full or partial agonists. The average maximal stimulation by serotonin was 565 ± 46 nM IP1. n=3–8. B. HEK-5-HT2C cells. All compounds are full agonists. The average maximal stimulation by serotonin was 1390 ± 180 nM. n=4–7.
Similar articles
- Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors.
Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A. Eshleman AJ, et al. Biochem Pharmacol. 2018 Dec;158:27-34. doi: 10.1016/j.bcp.2018.09.024. Epub 2018 Sep 25. Biochem Pharmacol. 2018. PMID: 30261175 Free PMC article. - Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs).
Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME. Rickli A, et al. Neuropharmacology. 2015 Dec;99:546-53. doi: 10.1016/j.neuropharm.2015.08.034. Epub 2015 Aug 25. Neuropharmacology. 2015. PMID: 26318099 - Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats.
Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Fantegrossi WE, et al. Psychopharmacology (Berl). 2005 Sep;181(3):496-503. doi: 10.1007/s00213-005-0009-4. Epub 2005 Oct 12. Psychopharmacology (Berl). 2005. PMID: 15983786 - Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens.
Halberstadt AL. Halberstadt AL. Curr Top Behav Neurosci. 2017;32:283-311. doi: 10.1007/7854_2016_64. Curr Top Behav Neurosci. 2017. PMID: 28097528 Review. - Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signaling.
Liechti M. Liechti M. Swiss Med Wkly. 2015 Jan 14;145:w14043. doi: 10.4414/smw.2015.14043. eCollection 2015. Swiss Med Wkly. 2015. PMID: 25588018 Review.
Cited by
- Population pharmacokinetic/pharmacodynamic modeling of the psychedelic experience induced by N,N-dimethyltryptamine - Implications for dose considerations.
Eckernäs E, Timmermann C, Carhart-Harris R, Röshammar D, Ashton M. Eckernäs E, et al. Clin Transl Sci. 2022 Dec;15(12):2928-2937. doi: 10.1111/cts.13410. Epub 2022 Sep 27. Clin Transl Sci. 2022. PMID: 36088656 Free PMC article. - Crayfish Learning: Addiction and the Ganglionic Brain.
van Staaden MJ, Huber R. van Staaden MJ, et al. Perspect Behav Sci. 2018 Nov 7;41(2):417-429. doi: 10.1007/s40614-018-00181-z. eCollection 2018 Nov. Perspect Behav Sci. 2018. PMID: 31976403 Free PMC article. - Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter.
Kozell LB, Eshleman AJ, Swanson TL, Bloom SH, Wolfrum KM, Schmachtenberg JL, Olson RJ, Janowsky A, Abbas AI. Kozell LB, et al. J Pharmacol Exp Ther. 2023 Apr;385(1):62-75. doi: 10.1124/jpet.122.001454. Epub 2023 Jan 20. J Pharmacol Exp Ther. 2023. PMID: 36669875 Free PMC article. - Population Survey Data Informing the Therapeutic Potential of Classic and Novel Phenethylamine, Tryptamine, and Lysergamide Psychedelics.
Sexton JD, Nichols CD, Hendricks PS. Sexton JD, et al. Front Psychiatry. 2020 Feb 11;10:896. doi: 10.3389/fpsyt.2019.00896. eCollection 2019. Front Psychiatry. 2020. PMID: 32116806 Free PMC article. - Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors.
Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A. Eshleman AJ, et al. Biochem Pharmacol. 2018 Dec;158:27-34. doi: 10.1016/j.bcp.2018.09.024. Epub 2018 Sep 25. Biochem Pharmacol. 2018. PMID: 30261175 Free PMC article.
References
- Backstrom JR, Chang MS, Chu H, Niswender CM, Sanders-Bush E. Agonist-directed signaling of serotonin 5-HT2C receptors: differences between serotonin and lysergic acid diethylamide (LSD) Neuropsychopharmacology. 1999;21:77S–81S. - PubMed
- Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, West WB, Appel JB. Assessment of the discriminative stimulus effects of the optical isomers of ecstasy (3,4-methylenedioxymethamphetamine; MDMA) Behav Pharmacol. 1995;6:263–275. - PubMed
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K05 DA000101/DA/NIDA NIH HHS/United States
- N01DA2-8822/DA/NIDA NIH HHS/United States
- Y01 DA005007/DA/NIDA NIH HHS/United States
- N01DA-7-8872/DA/NIDA NIH HHS/United States
- P50 DA018165/DA/NIDA NIH HHS/United States
- Y1-DA-0101-01/DA/NIDA NIH HHS/United States
- Y1 DA 5007-05/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources