The emerging role of the Nrf2-Keap1 signaling pathway in cancer - PubMed (original) (raw)
Review
The emerging role of the Nrf2-Keap1 signaling pathway in cancer
Melba C Jaramillo et al. Genes Dev. 2013.
Abstract
The Nrf2 (nuclear factor erythroid 2 [NF-E2]-related factor 2 [Nrf2])-Keap1 (Kelch-like erythroid cell-derived protein with CNC homology [ECH]-associated protein 1) signaling pathway is one of the most important cell defense and survival pathways. Nrf2 can protect cells and tissues from a variety of toxicants and carcinogens by increasing the expression of a number of cytoprotective genes. As a result, several Nrf2 activators are currently being tested as chemopreventive compounds in clinical trials. Just as Nrf2 protects normal cells, studies have shown that Nrf2 may also protect cancer cells from chemotherapeutic agents and facilitate cancer progression. Nrf2 is aberrantly accumulated in many types of cancer, and its expression is associated with a poor prognosis in patients. In addition, Nrf2 expression is induced during the course of drug resistance. Collectively, these studies suggest that Nrf2 contributes to both intrinsic and acquired chemoresistance. This discovery has opened up a broad spectrum of research geared toward a better understanding of the role of Nrf2 in cancer. This review provides an overview of (1) the Nrf2-Keap1 signaling pathway, (2) the dual role of Nrf2 in cancer, (3) the molecular basis of Nrf2 activation in cancer cells, and (4) the challenges in the development of Nrf2-based drugs for chemoprevention and chemotherapy.
Keywords: ARE; Keap1; Nrf2; chemoprevention; chemoresistance; oxidative stress.
Figures
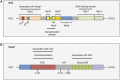
Figure 1.
Conserved domains of Nrf2 and Keap1. (A) Nrf2 contains seven domains, known as Neh1–Neh7. The Neh2 domain contains two binding motifs, DLG and ETGE, which interact with Keap1. The Neh4, Neh5, and Neh3 domains are important for the transactivation activity of Nrf2. The Neh6 domain is a serine-rich region that regulates Nrf2 stability. The Neh1 domain is a basic region leucine zipper motif that is important for its stability, DNA binding, and dimerization with Maf. (B) Keap1 contains three major domains. The BTB domain mediates Keap1 homodimerization and associates with Cul3. The IVR domain contains critical cysteine residues and connects the BTB domain with the C terminus Kelch/DGR domain. The Kelch/DGR domain mediates binding with the Neh2 domain of Nrf2.
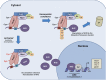
Figure 2.
Schematic model of the Nrf2–Keap1 signaling pathway. Under basal conditions, Keap1 binds to the ETGE and DLG motifs on Nrf2 and brings Nrf2 into Keap1–Cul3–E3 ubiquitin ligase complex, leading to ubiquitination and subsequent degradation of Nrf2. Oxidative stress or electrophiles can cause a conformational change in the Keap1–Cul3–E3 ubiquitin ligase by acting on specific cysteine residues in Keap1. These changes disrupt Nrf2–Keap1 binding at the DLG domain. Nrf2 is stabilized, and free Nrf2 translocates to the nucleus, where it dimerizes with members of the small Maf family and binds to AREs (5′-RTGABNNNGCR-3′) within regulatory regions of a wide variety of cell defense genes, including NQO1, GCLM, HO-1, and MRP1. (E) ETGE; (D) DLG.
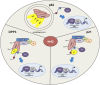
Figure 3.
Cross-talk between Nrf2 and other proteins. The substrate adaptor sequestosome 1 protein (p62) modulates the Nrf2–Keap1 signaling pathway by directly interacting with Keap1. p62 sequesters Keap1 in autophagosomes, which results in a decrease in Nrf2 ubiquitination, an increase in Nrf2 stability, and activation of Nrf2 target genes. Nrf2 is stabilized in response to p21 up-regulation. p21 associates with the DLG motif on Nrf2, thereby disrupting the ability of Keap1 to properly bind and ubiquitinate Nrf2. DPP3 binds to Keap1, inhibits Nrf2 ubiquitination, and drives Nrf2-dependent transcription in cancer cells. (E) ETGE; (S) STGE; (D) DLG.
Similar articles
- Role of the Keap1-Nrf2 pathway in cancer.
Leinonen HM, Kansanen E, Pölönen P, Heinäniemi M, Levonen AL. Leinonen HM, et al. Adv Cancer Res. 2014;122:281-320. doi: 10.1016/B978-0-12-420117-0.00008-6. Adv Cancer Res. 2014. PMID: 24974185 Review. - Immunohistochemical study of the Nrf2 pathway in colorectal cancer: Nrf2 expression is closely correlated to Keap1 in the tumor and Bach1 in the normal tissue.
Chang LC, Fan CW, Tseng WK, Chen JR, Chein HP, Hwang CC, Hua CC. Chang LC, et al. Appl Immunohistochem Mol Morphol. 2013 Dec;21(6):511-7. doi: 10.1097/PAI.0b013e318282ac20. Appl Immunohistochem Mol Morphol. 2013. PMID: 23455180 - Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer.
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Singh A, et al. PLoS Med. 2006 Oct;3(10):e420. doi: 10.1371/journal.pmed.0030420. PLoS Med. 2006. PMID: 17020408 Free PMC article. - Regulatory role of KEAP1 and NRF2 in PPARγ expression and chemoresistance in human non-small-cell lung carcinoma cells.
Zhan L, Zhang H, Zhang Q, Woods CG, Chen Y, Xue P, Dong J, Tokar EJ, Xu Y, Hou Y, Fu J, Yarborough K, Wang A, Qu W, Waalkes MP, Andersen ME, Pi J. Zhan L, et al. Free Radic Biol Med. 2012 Aug 15;53(4):758-68. doi: 10.1016/j.freeradbiomed.2012.05.041. Epub 2012 Jun 7. Free Radic Biol Med. 2012. PMID: 22684020 Free PMC article. - Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution.
Taguchi K, Motohashi H, Yamamoto M. Taguchi K, et al. Genes Cells. 2011 Feb;16(2):123-40. doi: 10.1111/j.1365-2443.2010.01473.x. Genes Cells. 2011. PMID: 21251164 Review.
Cited by
- Hepatoblastoma modeling in mice places Nrf2 within a cancer field established by mutant β-catenin.
Comerford SA, Hinnant EA, Chen Y, Bansal H, Klapproth S, Rakheja D, Finegold MJ, Lopez-Terrada D, O'Donnell KA, Tomlinson GE, Hammer RE. Comerford SA, et al. JCI Insight. 2016 Oct 6;1(16):e88549. doi: 10.1172/jci.insight.88549. JCI Insight. 2016. PMID: 27734029 Free PMC article. - Ubiquitin ligases in cancer: Functions and clinical potentials.
Duan S, Pagano M. Duan S, et al. Cell Chem Biol. 2021 Jul 15;28(7):918-933. doi: 10.1016/j.chembiol.2021.04.008. Epub 2021 May 10. Cell Chem Biol. 2021. PMID: 33974914 Free PMC article. Review. - p62/Sequestosome-1, Autophagy-related Gene 8, and Autophagy in Drosophila Are Regulated by Nuclear Factor Erythroid 2-related Factor 2 (NRF2), Independent of Transcription Factor TFEB.
Jain A, Rusten TE, Katheder N, Elvenes J, Bruun JA, Sjøttem E, Lamark T, Johansen T. Jain A, et al. J Biol Chem. 2015 Jun 12;290(24):14945-62. doi: 10.1074/jbc.M115.656116. Epub 2015 Apr 30. J Biol Chem. 2015. PMID: 25931115 Free PMC article. - NRF2 and glutathione are key resistance mediators to temozolomide in glioma and melanoma cells.
Rocha CR, Kajitani GS, Quinet A, Fortunato RS, Menck CF. Rocha CR, et al. Oncotarget. 2016 Jul 26;7(30):48081-48092. doi: 10.18632/oncotarget.10129. Oncotarget. 2016. PMID: 27344172 Free PMC article. - Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer.
Walerych D, Lisek K, Sommaggio R, Piazza S, Ciani Y, Dalla E, Rajkowska K, Gaweda-Walerych K, Ingallina E, Tonelli C, Morelli MJ, Amato A, Eterno V, Zambelli A, Rosato A, Amati B, Wiśniewski JR, Del Sal G. Walerych D, et al. Nat Cell Biol. 2016 Aug;18(8):897-909. doi: 10.1038/ncb3380. Epub 2016 Jun 27. Nat Cell Biol. 2016. PMID: 27347849
References
- Adam J, Hatipoglu E, O'Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. 2011. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: Roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20: 524–537 - PMC - PubMed
- Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG 2004. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr 134: 1134–1138 - PubMed
- Bartel DP 2004. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281–297 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES006694/ES/NIEHS NIH HHS/United States
- R01 CA154377/CA/NCI NIH HHS/United States
- ES015010/ES/NIEHS NIH HHS/United States
- R01 ES015010/ES/NIEHS NIH HHS/United States
- P30ES006694/ES/NIEHS NIH HHS/United States
- T32 ES007091/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials