Structural and mechanistic insight into DNA unwinding by Deinococcus radiodurans UvrD - PubMed (original) (raw)
Structural and mechanistic insight into DNA unwinding by Deinococcus radiodurans UvrD
Meike Stelter et al. PLoS One. 2013.
Abstract
DNA helicases are responsible for unwinding the duplex DNA, a key step in many biological processes. UvrD is a DNA helicase involved in several DNA repair pathways. We report here crystal structures of Deinococcus radiodurans UvrD (drUvrD) in complex with DNA in different nucleotide-free and bound states. These structures provide us with three distinct snapshots of drUvrD in action and for the first time trap a DNA helicase undergoing a large-scale spiral movement around duplexed DNA. Our structural data also improve our understanding of the molecular mechanisms that regulate DNA unwinding by Superfamily 1A (SF1A) helicases. Our biochemical data reveal that drUvrD is a DNA-stimulated ATPase, can translocate along ssDNA in the 3'-5' direction and shows ATP-dependent 3'-5', and surprisingly also, 5'-3' helicase activity. Interestingly, we find that these translocase and helicase activities of drUvrD are modulated by the ssDNA binding protein. Analysis of drUvrD mutants indicate that the conserved β-hairpin structure of drUvrD that functions as a separation pin is critical for both drUvrD's 3'-5' and 5'-3' helicase activities, whereas the GIG motif of drUvrD involved in binding to the DNA duplex is essential for the 5'-3' helicase activity only. These special features of drUvrD may reflect its involvement in a wide range of DNA repair processes in vivo.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
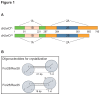
Figure 1. Domain organization of _dr_UvrD and structure of the various DNA oligonucleotides used for crystallization.
A. Schematic representation of the domain structures of _dr_UvrDFL and _dr_UvrD∆C. B. Structure of DNA oligonucleotides used for crystallization with _dr_UvrDFL and _dr_UvrD∆C. The circles represent UvrD bound to the DNA as observed in our crystal structures.
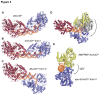
Figure 2. Structure of the _dr_UvrD helicase.
A. Crystal structure of one monomer of _dr_UvrD∆C bound to duplex DNA with a single-stranded extension at the 3′-end. The translocating strand is colored black and the complementary strand is colored red. The domains of _dr_UvrD∆C are shown in ribbon and are colored green (1A), beige (1B), orange (a) and blue (2B). AMPPNP is shown in sticks. B. Overlay of nucleotide-bound _dr_UvrD (blue) and _ec_UvrD (grey) structures. The main structural difference is the linker between domains 2B and 2A that adopts a helical arrangement in _dr_UvrD (α25) as opposed to a flexible coil in _ec_UvrD.
Figure 3. Crystal structures of _dr_UvrD-DNA complexes.
A ribbon illustration of the AMPPNP-bound _dr_UvrDFL is shown in A, the AMPPNP-bound _dr_UvrD∆C form I is shown in B, the mixed AMPPNP-bound (red) and apo- (blue) _dr_UvrD∆C form II is shown in C. The DNA and AMPPNP are shown in sticks. D-E. Large-scale conformational changes. D. Overlay of chains A (red) of _dr_UvrDFL, _dr_UvrD∆C form I and apo-_dr_UvrD∆C form II, illustrating the large spiral movement of chains B colored respectively yellow, grey and blue. The DNA is shown as an orange ribbon. E. As in (D) but viewed down the DNA axis, and for clarity _dr_UvrD∆C form I has been removed.
Figure 4. Conformational changes associated with ATP hydrolysis and nucleotide release.
A-C. Domain movements. The AMPPNP-bound form is colored in red, while the apo-form is colored in blue. A. Upon ATP hydrolysis and nucleotide release, domain 2B along with the dsDNA rotates by ~15° and domain 1A and 1B by 8° relative to domain 2A. B. Close up view of the rotation of domain 2B and duplex DNA. C. Domains 1A and 1B undergo a 15° twist relative to domain 2A around the ssDNA axis (orange). D. Conformational changes occurring at the ssDNA gateway (circled in green). The linker between domains 2B and 2A adopts a short helix (α25) and loop in the AMPPNP-bound form and interacts tightly with the 3′-end of the ssDNA via Ser546, while it consists of an unstructured loop (dashed line) in the apo-form. In the AMPPNP form, the ssDNA gateway is more closed: the distance between the carboxyl oxygen of Phe65 (motif Ia) and the hydroxyl group of Ser546 is 4.5 Å in the AMPPNP-bound form versus 9.9 Å in the apo-form. The represented DNA corresponds to the AMPPNP bound form.
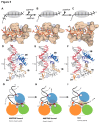
Figure 5. DNA binding of _dr_UvrD.
Illustrations of _dr_UvrD binding to dsDNA with a 3′-ssDNA tail in form I (A,D and G), form II with AMPPNP bound (B, E and H) and in the apo-form of form II (C, F and I). A-C. Schematic diagrams (top) illustrating the translocation of form I (A), form II with AMPPNP bound (B) and the apo-form of form II (C) of _dr_UvrD∆C along the ssDNA. The ssDNA nucleotides are illustrated as black bars and are numbered as in the crystal structures. The grey oval shape representing _dr_UvrD covers the nucleotides bound in the ssDNA binding pocket. Surface representations of the ssDNA binding pockets of these three forms of _dr_UvrD∆C bound to ssDNA (orange sticks) are shown below. The important residues are labeled and the bases are numbered as in the schematic diagrams. D-F. Binding of _dr_UvrD∆C to dsDNA in form I (D), form II with AMPPNP bound (E) and in the apo-form of form II (F). The dsDNA is illustrated in sticks with the translocated strand in grey. Domains of _dr_UvrD are colored as in Figure 2A. The helices belonging to the HLH motifs and the β-hairpin structure (orange) are shown and labeled according to the secondary structure succession (Figure S1). The positively charged residues in contact with dsDNA are illustrated in sticks and the GIG motif is indicated. The number of base-pairs formed between the ss-dsDNA junction and the contact point with the _dr_UvrD GIG motif is shown to the left of each panel. This number differs significantly between the two crystal forms. G-I. Schematic representation of _dr_UvrD's DNA binding in the different crystal structures as indicated below the models. The four protein-DNA contact points that are critical for the wrench-and-inchworm unwinding mechanism are indicated with circled numbers in all panels: HLH motifs interact with dsDNA (1), the β-hairpin motif with the ss-dsDNA junction (2), motif III with the ssDNA (3) and the ssDNA gateway with the exiting ssDNA (4). G. In AMPPNP bound Form I, contact points 1, 3 and 4 are tight. H. In AMPPNP bound Form II, _dr_UvrD's GIG motif (1) has slided along the DNA duplex and pushes the DNA junction against the β-hairpin motif (2), which now stacks tightly against the first base-pair. I. In the apo molecule of Form II, the ssDNA gateway (4) has opened and ssDNA exited the helicase. Domains of _dr_UvrD are colored as in Figure 2A.
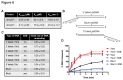
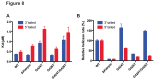
Figure 6. ATPase and helicase activity of _dr_UvrD.
A. DNA-stimulated ATPase kinetic parameters of _dr_UvrDFL and _dr_UvrD∆C. B. Structure of DNA oligonucleotides used for helicase assay of _dr_UvrD. The fluorescein label is represented as a star. C.-D. Helicase activity of _dr_UvrDFL on DNA substrates shown in (B). C. Table summarising the initial rates of unwinding of duplexed DNA containing 15 or 7 nucleotide ssDNA extensions at either the 3′ or 5′ ends and of blunt duplexed DNA, as indicated, and in the absence and presence of _dr_SSB (250 nM). The rates are given in base-pairs per min per UvrD helicase unit (bp/min/UvrD). D. Time course of _dr_UvrD unwinding of duplexed DNA containing 15 nucleotide ssDNA extensions at either the 3′ (red) or 5′ (black)-ends and of blunt (blue) duplexed DNA in the absence (full line) and presence (dotted line) of _dr_SSB (250 nM). Standard deviations are shown as vertical bars.
Figure 7. ssDNA translocase activity of _dr_UvrD.
Translocase activity of _dr_UvrD was assayed using the streptavidin-displacement assay. A. Structure of DNA oligonucleotides used for _dr_UvrD translocase assay measuring streptavidin displacement from biotinylated DNA substrates. The fluorescein label is represented as a star and the biotin label as a circle. B. Time course of _dr_UvrD (250 nM) catalyzed streptavidin displacement from the 3′- (blue) and 5′- (red) ssDNA extensions of DNA oligonucleotides shown in (A). The fraction of released dsDNA (no longer bound to streptavidin) was quantified and plotted as a function of time. C. Translocase activity of _dr_UvrD (250 nM) on 5' tailed dsDNA (20 nM) as a function of time in the absence (left) and the presence (right) of _dr_SSB (250 nM). The reaction products were analyzed on a 10 % polyacrylamide TBE gel. Bands correspond to the fluorescein labeled reaction products: streptavidin-bound dsDNA (upper bands, corresponding to several biotin labeled oligonucleotides bound to streptavidin), released dsDNA (middle band) and unwound ssDNA (lower band). D. The bands shown in (C), resulting from the time course of streptavidin displacement from 5′- tailed dsDNA, were quantified and the fraction of streptavidin-bound (black), released dsDNA (red) and unwound ssDNA (blue) were plotted as a function of time for reactions carried out in the absence (full lines) and presence (dotted lines) of _dr_SSB (250 nM). Standard deviations are shown as vertical bars.
Figure 8. DNA binding ability and helicase activity of drUvrD mutants.
Comparison of DNA binding ability and helicase activity of wild type (WT) and _dr_UvrD mutants: β-hairpin deletion mutant (ΔHairpin), and mutants of the GIG motif from domain 2B involved in dsDNA binding (G424T, G426T and double mutant G424T/G426T). A. DNA binding affinities (Kd values) of WT and mutant _dr_UvrD for either 3'-tailed (blue) or 5'-tailed (red) dsDNA determined by fluorescence anisotropy measurements. B. Helicase activity of WT and mutant _dr_UvrD (250 nM) on 3'-tailed (blue) or 5'-tailed (red) dsDNA (20 nM). Initial reaction rates were determined from reaction time courses and were normalized with respect to the activity of WT _dr_UvrD. Standard deviations are shown as vertical bars.
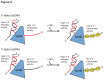
Figure 9. Model of DNA duplex unwinding and ssDNA translocation by _dr_UvrD.
Models of _dr_UvrD DNA unwinding and ssDNA translocase activity on 5' tailed dsDNA (top) and 3' tailed dsDNA (bottom) in the absence (left) and presence (right) of _dr_SSB. Using 5' tailed dsDNA, in the absence of _dr_SSB _dr_UvrD has low 5'-3' helicase activity and high 3'-5' translocase activity while, in the presence of _dr_SSB, _dr_UvrD has high helicase activity and low translocase activity. Using 3'-tailed dsDNA, _dr_UvrD has high 3'-5' helicase activity and no 5'-3' translocase activity, regardless of the absence or presence of _dr_SSB.
Similar articles
- Mechanistic basis of 5'-3' translocation in SF1B helicases.
Saikrishnan K, Powell B, Cook NJ, Webb MR, Wigley DB. Saikrishnan K, et al. Cell. 2009 May 29;137(5):849-59. doi: 10.1016/j.cell.2009.03.036. Cell. 2009. PMID: 19490894 - An oligomeric form of E. coli UvrD is required for optimal helicase activity.
Ali JA, Maluf NK, Lohman TM. Ali JA, et al. J Mol Biol. 1999 Nov 5;293(4):815-34. doi: 10.1006/jmbi.1999.3185. J Mol Biol. 1999. PMID: 10543970 - Mechanism of ATP-dependent translocation of E.coli UvrD monomers along single-stranded DNA.
Fischer CJ, Maluf NK, Lohman TM. Fischer CJ, et al. J Mol Biol. 2004 Dec 10;344(5):1287-309. doi: 10.1016/j.jmb.2004.10.005. J Mol Biol. 2004. PMID: 15561144 - Non-hexameric DNA helicases and translocases: mechanisms and regulation.
Lohman TM, Tomko EJ, Wu CG. Lohman TM, et al. Nat Rev Mol Cell Biol. 2008 May;9(5):391-401. doi: 10.1038/nrm2394. Nat Rev Mol Cell Biol. 2008. PMID: 18414490 Review. - Insights into the Dynamics and Helicase Activity of RecD2 of Deinococcus radiodurans during DNA Repair: A Single-Molecule Perspective.
Islam F, Purkait D, Mishra PP. Islam F, et al. J Phys Chem B. 2023 May 25;127(20):4351-4363. doi: 10.1021/acs.jpcb.3c00778. Epub 2023 May 10. J Phys Chem B. 2023. PMID: 37163679 Review.
Cited by
- DNA Damage Protection for Enhanced Bacterial Survival Under Simulated Low Earth Orbit Environmental Conditions in Escherichia coli.
Puig J, Knödlseder N, Quera J, Algara M, Güell M. Puig J, et al. Front Microbiol. 2021 Dec 14;12:789668. doi: 10.3389/fmicb.2021.789668. eCollection 2021. Front Microbiol. 2021. PMID: 34970246 Free PMC article. - Rep and UvrD Antagonize One Another at Stalled Replication Forks and This Is Exacerbated by SSB.
Liu X, Seet JX, Shi Y, Bianco PR. Liu X, et al. ACS Omega. 2019 Mar 31;4(3):5180-5196. doi: 10.1021/acsomega.8b02375. Epub 2019 Mar 12. ACS Omega. 2019. PMID: 30949615 Free PMC article. - Molecular basis for RNA polymerase-dependent transcription complex recycling by the helicase-like motor protein HelD.
Newing TP, Oakley AJ, Miller M, Dawson CJ, Brown SHJ, Bouwer JC, Tolun G, Lewis PJ. Newing TP, et al. Nat Commun. 2020 Dec 18;11(1):6420. doi: 10.1038/s41467-020-20157-5. Nat Commun. 2020. PMID: 33339820 Free PMC article. - A Decade of Biochemical and Structural Studies of the DNA Repair Machinery of Deinococcus radiodurans: Major Findings, Functional and Mechanistic Insight and Challenges.
Timmins J, Moe E. Timmins J, et al. Comput Struct Biotechnol J. 2016 Apr 7;14:168-176. doi: 10.1016/j.csbj.2016.04.001. eCollection 2016. Comput Struct Biotechnol J. 2016. PMID: 27924191 Free PMC article. Review. - Structural features and functional implications of proteins enabling the robustness of Deinococcus radiodurans.
Chen Z, Tang Y, Hua Y, Zhao Y. Chen Z, et al. Comput Struct Biotechnol J. 2020 Oct 7;18:2810-2817. doi: 10.1016/j.csbj.2020.09.036. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33133422 Free PMC article. Review.
References
- Gorbalenya AE, Koonin EV (1993) Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol 3: 419-429. doi:10.1016/S0959-440X(05)80116-2. - DOI
Publication types
MeSH terms
Substances
Grants and funding
This work was funded by the inhouse research program of the ESRF. MS and JT are funded by the CNRS via an ATIP-AVENIR grant and by the Ligue contre le Cancer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources