Binding of a sialic acid-recognizing lectin Siglec-9 modulates adhesion dynamics of cancer cells via calpain-mediated protein degradation - PubMed (original) (raw)
Binding of a sialic acid-recognizing lectin Siglec-9 modulates adhesion dynamics of cancer cells via calpain-mediated protein degradation
Ilhamjan Sabit et al. J Biol Chem. 2013.
Abstract
Although regulatory mechanisms for immune cells with inhibitory signals via immunoreceptor tyrosine-based inhibitory motifs are well known, signals transduced via interaction between Siglecs and sialyl compounds on their counterreceptors into target cells have not been reported to date. In this study, we found that an astrocytoma cell line, AS, showed detachment from culture plates when co-cultured with Siglec-9-expressing cells and/or soluble Siglec-9. Moreover, detached AS cells regrew as co-cultured cells with Siglec-9-deficient cells. They also showed increased motility and invasiveness upon Siglec-9 binding. In immunoblotting, rapid degradation of focal adhesion kinase (FAK) and related signaling molecules such as Akt, paxillin, and p130Cas was observed immediately after the co-culture. Despite degradation of these molecules, increased p-Akt was found at the front region of the cytoplasm, probably reflecting increased cell motility. Calpain was considered to be a responsible protease for the protein degradation by the inhibition experiments. These results suggest that protein degradation of FAK and related molecules was induced by Siglec-9 binding to its counterreceptors via sialylglycoconjugates, leading to the modulation of adhesion kinetics of cancer cells. Thus, this might be a mechanism by which cancer cells utilize Siglec-9-derived signals to escape from immunosurveillance.
Keywords: Adhesion; Calpain; Focal Adhesion Kinase; Lectin; Protein Degradation; Sialic Acid.
Figures
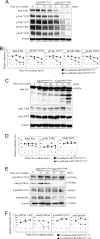
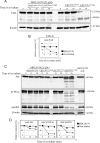
FIGURE 1.
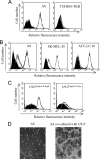
Cancer cells expressing ligands for Siglec-9 and effector cells expressing Siglec-9 on the cell surface. A, 75 human cell lines were screened for binding of Siglec-9Fc by flow cytometry. Representative examples (AS, astrocytoma; TUHR10-TKB, renal cell cancer) are shown. B, glycans recognized by Siglec-9 were characterized using neuraminidase. Siglec-9 binding reduced after treatment with neuramidase (200 milliunits/ml) in three cell lines. Definite reduction in the binding intensities of Siglec-9Fc was observed in AS cells (93.4%; mean fluoresence intensity (MFI) 345.1 versus 22.7), SK-MEL-28 (melanoma) (95.5%; MFI 284.3 versus 12.7), and SK-LC-10 (lung cancer) (98.9%; MFI 332.7 versus 3.6). C, U937Siglec-9-high and U937Siglec-9-low clones were used in the co-culture system. Full length of Siglec-9 cDNA was transfected into U937 and sorted by labeling with anti-human Siglec-9 antibody using FACSAria IITM, resulting in the establishment of U937Siglec-9-high and U937Siglec-9-low. D, morphological features of these cells are shown (magnification, ×200).
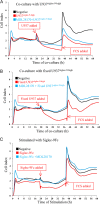
FIGURE 2.
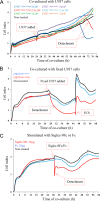
RT-CES of AS cells co-cultured with U937Siglec-9-high and U937Siglec-9-low or treated with soluble Siglec-9Fc. A, CI of AS cells (1 × 104 cells) co-cultured with U937 cells (10,000, 25,000, and 50,000 cells). B, CI of AS cells co-cultured with U937 cells fixed in ethanol:acetic acid. One × 104 AS cells were maintained in RPMI 1640 medium containing 10% FCS, then U937 cells (1 × 105) were added (final FCS 3.5%). When FCS was added up to 10%, U937Siglec-9-high-treated AS cells reattached and regrew. C, CI of AS cells treated with Siglec-9Fc or Fc proteins (150 μg/ml). All samples were analyzed and duplicated, and average values of similar results (<5% differences) are shown. These experiments were repeated at least three times with similar results.
FIGURE 3.
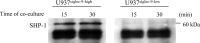
Immunoblotting of SHP-1. Recruitment of SHP-1 in U937 cells co-cultured with AS cells was examined at 15 and 30 min. U937Siglec-9-high and U937Siglec-9-low co-cultured with AS cells for 15 and 30 min were lysed, then 300 μg of the lysates was precipitated with 2 μg of anti-human Siglec-9 antibody (R&D Systems) and protein G-Sepharose (GE Healthcare). The precipitates were immunoblotted by anti-SHP-1 antibody. No recruitment of SHP-1 was found in U937Siglec-9-low cells, whereas definite bands were detected in U937Siglec-9-high cells. This experiment was repeated at least three times with similar results.
FIGURE 4.
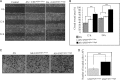
Effects of co-culture on AS cell properties. A, wound healing scratching assay performed as described under “Experimental Procedures.” Representative results of AS cell migration are shown (magnification, ×100) at time 0, 12, and 24 h co-culture. B, results in A are presented as filled areas (%). Untreated AS cells (control) covered 37.7% (12 h) and 57% (24 h). AS cells co-cultured with U937Siglec-9-low showed similar results. AS cells co-cultured with U937Siglec-9-high showed significantly higher motility than these two (***, p < 0.01). C, invasion of AS cells during co-culture. AS cell invasion activity in a Matrigel-coated Boyden chamber was measured. Representative images of stained cells are shown (magnification, ×200). Invasion of AS cells increased after co-culture with U937Siglec-9-high for 24 h compared with those co-cultured with U937Siglec-9-low (n = 10; ***, p < 0.01). Error bars, S.E.
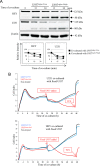
FIGURE 5.
Degradation of FAK, Akt, p130Cas, and paxillin in AS cells during co-culture with U937Siglec-9-high. AS cells were co-cultured with U937 cells for 5, 15, and 30 min, and total protein samples were harvested. Fifteen μg of proteins was tested by immunoblotting. A, results of immunoblotting for total FAK and phosphorylated FAK at Tyr-576, Tyr-577, Tyr-861, and Tyr-925 in AS cells during co-culture with U937 cells. β-Actin was used for loading control. B, results of the densitometric analysis of total FAK and phosphorylated FAKs. Results are shown as expression and/or phosphorylation levels of FAK after normalization with band intensities of β-actin. C, results of immunoblotting for total Akt and phosphorylated forms at Thr-308 and Ser-473 in AS cells treated as in A. D, results of the densitometric analysis of total Akt and phosphorylated Akts. Results are shown as described in B. E, results of immunoblotting for p130Cas and paxillin in AS cells treated as in A and C. F, results of the densitometric analysis of total p130Cas and paxillin and phosphorylated forms as shown in B and D. These experiments were repeated at least three times with similar results.
FIGURE 6.
Degradation of FAK and related molecules by addition of Siglec-9Fc. A–D, 5 ×104 AS cells were seeded in 6-well plates and cultured in 10% FCS-containing RPMI 1640 medium for 24 h. Then, the medium was exchanged with FCS-free medium, and cells were incubated for 12 h. Siglec-9Fc or Fc proteins (20 μg) were then added to the wells. After a 15- and 30-min incubation, AS cells were harvested for immunoblotting. Fifteen μg of each protein was applied to 8% SDS-PAGE and then wet transferred onto PVDF membrane, and detected by anti-FAK (A), anti-Akt (B), anti-p130Cas (C) and anti-paxillin (D) antibodies as described in Fig. 5. E, effects of Siglec-9Fc cross-linked by anti-human IgG Fc secondary antibody are shown. To AS cells prepared as in A, preformed Siglec-9Fc and secondary antibody complex (5 or 20 μg each) was added. Anti-human IgG F(ab′)2 fragment (Sigma) was used by mixing in 200 μl of PBS before incubation for 2 h at room temperature. Fc fusion protein instead of Siglec-9Fc was used as a negative control. After a 15- and 30-min incubation, AS cells were washed and harvested for immunoblotting. Fifteen μg of each protein was applied to 8% SDS-PAGE, then detected by anti-FAK (N-terminal) antibody. *, nonspecific band; deg, degradation products.
FIGURE 7.
Restoration of FAK and Akt and phosphorylation of Akt Thr-308 in AS cells treated with 10% FCS after co-culture. Co-culture with U937Siglec-9-high cells was performed as described in Fig. 5. 10% FCS was then added to culture medium after 30 min of co-culture following removal of U937 Siglec-9-high cells (left) or in the presence of U937Siglec-9-high cells (right), and then incubated up to 24 h. At the individual time points, AS cells were harvested, then cell lysates were applied for immunoblotting for FAK (A), and Akt (total Akt (lower), and p-Akt (upper)) (B) as described in the legend for Fig. 5. *, nonspecific band; Degrad., degradation products.
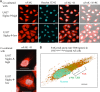
FIGURE 8.
FAK degradation during co-culture shown by immunocytochemistry. A, 6 ×104 AS cells were seeded in glass-based dishes (Iwaki) and cultured for 1–2 days, then FCS was starved for 12 h. AS cells were co-cultured with 1 × 106 U937Siglec-9-high or U937Siglec-9-low cells. After co-culture for 30 min, U937 cells were removed by washing with plain medium. Cells were fixed with 4% paraformaldehyde, then permeabilized with 0.1% Triton X-100/PBS (pH 7.2). After being blocked with 5% BSA/PBS for 1 h, cells were stained with anti-FAK antibody at 1:400 dilution for 1 h at room temperature, then incubated with rabbit anti-mouse IgG Alexa Fluor 568 secondary antibody (red) (Invitrogen) at 1:400 dilution for 60 min in the dark. The cell nucleus was then stained with Hoechst 33342 (1:10,000 dilution) (light blue) for 15 min. Finally, cells were observed with FV10iTM Laser Scanning Confocal Microscope (Olympus, Japan). Cell images were analyzed by FV10-ASW ViewerTM (v3.01). Low magnification images (upper) and high magnification images (lower) are shown. Scale bars, 25 μm. B, cells were stained with anti-FAK antibody as in A and anti-p-Akt Thr-308 (rabbit IgG, at 1:400 dilution; Cell Signaling Technology) for 60 min at room temperature. Then, cells were stained with Alexa Fluor 555-goat anti-mouse IgG1 for FAK (1:400 dilution; Invitrogen) and Alexa Fluor 488-goat anti-rabbit IgG (H+L) antibody (Invitrogen) for p-Akt Thr-308. Cells were also stained with Hoechst 33342. Details for image analysis and individual images are shown in
supplemental Fig. S4
. The co-staining pattern of FAK, p-Akt, and nucleus in U937Siglec-9-high-treated AS cells is shown. Scale bars, 10 μm.
FIGURE 9.
Degradation of FAK in HEY (ovarian cancer) and U251 (astrocytoma) cells during co-culture with U937Siglec-9-high. HEY and U251 cells were co-cultured with U937 cells as described above for AS cells. A, results of immunoblotting for FAK. Anti-FAK (N terminus) antibody was used (upper). Note that strong FAK degradation was observed during co-culture with U937Siglec-9-high as observed in AS cells. Results of the densitometric analysis of FAK are shown after normalization with band intensities of β-actin as in Fig. 5 (lower). B, CI of U251 cells (upper) or HEY cells (lower) co-cultured with fixed U937 cells. One × 104 cells were maintained in RPMI 1640 medium containing 10% FCS, then U937 cells (1 × 105) were added (final FCS 3.5%). After 24 h, FCS was added up to 10% as indicated to examine the restoration of cell growth as in Fig. 2. These experiments were repeated at least three times with similar results.
FIGURE 10.
Calpain is responsible for the detachment of AS cells co-cultured with U937. MDL-28170 prevented cell detachment of AS cells during co-culture with U937Siglec-9-high. One × 104 AS cells were cultured for 20 h in 100 μl of the regular medium with or without MDL-28170 (25 μ
m
), then were co-cultured with 1 × 105 of U937Siglec-9-high (A), fixed U937Siglec-9-high (B), or Siglec-9-Fc/Fc proteins (150 μg/ml) (C). Note that MDL-28170 treatment prevented AS cells from detaching. These experiments were repeated at least three times with similar results.
FIGURE 11.
Complete protection of protein degradation with a calpain inhibitor, MDL-28170. A and C, effects of a calpain inhibitor on the protein degradation of FAK (A), Akt, p130Cas, and paxillin (C) were examined. A, AS cells were treated with MDL-28170 (25 μ
m
) and co-cultured and immunoblotted as described. B, band intensities in A were measured and presented after normalization with those of β-actin. C, degradation of Akt, p130Cas, and paxillin was also examined to analyze effects of MDL-28170 on their degradation in AS cells during co-culture with U937Siglec-9-high. Immunoblotting was performed as described above. D, results of the densitometric analysis of bands of Akt, p130Cas, and paxillin shown in C are presented after normalization with those of β-actin.
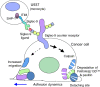
FIGURE 12.
Schema showing the results demonstrated in this paper.
Similar articles
- Viewing Siglecs through the lens of tumor immunology.
Fraschilla I, Pillai S. Fraschilla I, et al. Immunol Rev. 2017 Mar;276(1):178-191. doi: 10.1111/imr.12526. Immunol Rev. 2017. PMID: 28258691 Free PMC article. Review. - Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer.
Läubli H, Pearce OM, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, Deng L, Verhagen A, Secrest P, Lusk C, Schwartz AG, Varki NM, Bui JD, Varki A. Läubli H, et al. Proc Natl Acad Sci U S A. 2014 Sep 30;111(39):14211-6. doi: 10.1073/pnas.1409580111. Epub 2014 Sep 15. Proc Natl Acad Sci U S A. 2014. PMID: 25225409 Free PMC article. - Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth.
Tanida S, Akita K, Ishida A, Mori Y, Toda M, Inoue M, Ohta M, Yashiro M, Sawada T, Hirakawa K, Nakada H. Tanida S, et al. J Biol Chem. 2013 Nov 1;288(44):31842-52. doi: 10.1074/jbc.M113.471318. Epub 2013 Sep 17. J Biol Chem. 2013. PMID: 24045940 Free PMC article. - Sensing the neuronal glycocalyx by glial sialic acid binding immunoglobulin-like lectins.
Linnartz-Gerlach B, Mathews M, Neumann H. Linnartz-Gerlach B, et al. Neuroscience. 2014 Sep 5;275:113-24. doi: 10.1016/j.neuroscience.2014.05.061. Epub 2014 Jun 9. Neuroscience. 2014. PMID: 24924144 Review.
Cited by
- Cancer intelligence acquired (CIA): tumor glycosylation and sialylation codes dismantling antitumor defense.
Boligan KF, Mesa C, Fernandez LE, von Gunten S. Boligan KF, et al. Cell Mol Life Sci. 2015 Apr;72(7):1231-48. doi: 10.1007/s00018-014-1799-5. Epub 2014 Dec 7. Cell Mol Life Sci. 2015. PMID: 25487607 Free PMC article. Review. - Lectin galactoside-binding soluble 3 binding protein (LGALS3BP) is a tumor-associated immunomodulatory ligand for CD33-related Siglecs.
Läubli H, Alisson-Silva F, Stanczak MA, Siddiqui SS, Deng L, Verhagen A, Varki N, Varki A. Läubli H, et al. J Biol Chem. 2014 Nov 28;289(48):33481-91. doi: 10.1074/jbc.M114.593129. Epub 2014 Oct 15. J Biol Chem. 2014. PMID: 25320078 Free PMC article. - Viewing Siglecs through the lens of tumor immunology.
Fraschilla I, Pillai S. Fraschilla I, et al. Immunol Rev. 2017 Mar;276(1):178-191. doi: 10.1111/imr.12526. Immunol Rev. 2017. PMID: 28258691 Free PMC article. Review. - Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer.
Läubli H, Pearce OM, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, Deng L, Verhagen A, Secrest P, Lusk C, Schwartz AG, Varki NM, Bui JD, Varki A. Läubli H, et al. Proc Natl Acad Sci U S A. 2014 Sep 30;111(39):14211-6. doi: 10.1073/pnas.1409580111. Epub 2014 Sep 15. Proc Natl Acad Sci U S A. 2014. PMID: 25225409 Free PMC article. - Siglec-9, a Putative Immune Checkpoint Marker for Cancer Progression Across Multiple Cancer Types.
Wu Y, Huang W, Xie Y, Wang C, Luo N, Chen Y, Wang L, Cheng Z, Gao Z, Liu S. Wu Y, et al. Front Mol Biosci. 2022 Mar 17;9:743515. doi: 10.3389/fmolb.2022.743515. eCollection 2022. Front Mol Biosci. 2022. PMID: 35372497 Free PMC article.
References
- Crocker P. R., Paulson J. C., Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 - PubMed
- Angata T., Varki A. (2000) Cloning, characterization, and phylogenetic analysis of Siglec-9, a new member of the CD33-related group of Siglecs: evidence for co-evolution with sialic acid synthesis pathways. J. Biol. Chem. 275, 22127–22135 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous