CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure - PubMed (original) (raw)
CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure
Xingxu Wang et al. Mol Pharmacol. 2014 Jan.
Abstract
Prolonged endoplasmic reticulum (ER) stress causes apoptosis and is associated with heart failure. Whether CYP2J2 and its arachidonic acid metabolites [epoxyeicosatrienoic acids (EETs)] have a protective influence on ER stress and heart failure has not been studied. Assays of myocardial samples from patients with end-stage heart failure showed evidence of ER stress. Chronic infusion of isoproterenol (ISO) or angiotensin II (AngII) by osmotic mini-pump induced cardiac hypertrophy and heart failure in mice as evaluated by hemodynamic measurements and echocardiography. Interestingly, transgenic (Tr) mice with cardiomyocyte-specific CYP2J2 expression were protected against heart failure compared with wild-type mice. ISO or AngII administration induced ER stress and apoptosis, and increased levels of intracellular Ca(2+). These phenotypes were abolished by CYP2J2 overexpression in vivo or exogenous EETs treatment of cardiomyocytes in vitro. ISO or AngII reduced sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA2a) expression in hearts or isolated cardiomyocytes; however, loss of SERCA2a expression was prevented in CYP2J2 Tr hearts in vivo or in cardiomyocytes treated with EETs in vitro. The reduction of SERCA2a activity was concomitant with increased oxidation of SERCA2a. EETs reversed SERCA2a oxidation through increased expression of antioxidant enzymes and reduced reactive oxygen species levels. Tempol, a membrane-permeable radical scavenger, similarly decreased oxidized SERCA2a levels, restored SERCA2a activity, and markedly reduced ER stress response in the mice treated with ISO. In conclusion, CYP2J2-derived EETs suppress ER stress response in the heart and protect against cardiac failure by maintaining intracellular Ca(2+) homeostasis and SERCA2a expression and activity.
Figures
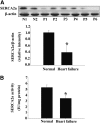
Fig. 1.
SERCA2a expression and activity were reduced in failing human hearts. (A) The expression of SERCA2a in normal (N1 and N2) and failing (P1–P6) human hearts is shown. Corresponding clinical characteristics of the six patients with heart failure are shown in Table 1. (B) SERCA2a activity was decreased in failing human hearts. Proteins were normalized to _β_-actin (normal human hearts, n = 4; failing human hearts, n = 6). *P < 0.05 versus normal hearts.
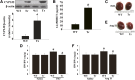
Fig. 2.
Attenuation of cardiac hypertrophy and dysfunction induced by ISO or AngII in CYP2J2 Tr mice. (A) CYP2J2 protein level was increased in CYP2J2 Tr mice compared with WT mice. (B) Urinary 14,15-DHET levels were increased in CYP2J2 Tr mice compared with WT mice (n = 10 per group). *P < 0.05 versus WT mice. (C and E) Representative gross appearance of hearts (left) from CYP2J2 Tr and WT mice treated with ISO or AngII, respectively. (D and F) The ratio of heart weight/body weight (HW/BW) of CYP2J2 Tr and WT mice treated with ISO or AngII, respectively (n = 5). *P < 0.05 versus WT mice treated with saline; #_P<_0.05 versus WT mice treated with ISO or AngII. Scale bar, 5 mm.
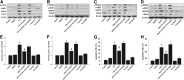
Fig. 3.
ER stress signaling was suppressed in CYP2J2 Tr mice. (A) ISO-induced ER stress and apoptosis was alleviated in CYP2J2 Tr mice. (B) Densitometric analysis of ER stress markers was shown (n = 5 per group). (C) AngII-induced ER stress and apoptosis were also inhibited in CYP2J2 Tr mice. (D) Densitometric analysis of ER stress markers was shown (n = 5 per group). (E) Representative images of TUNEL staining showing cardiac myocytes apoptosis and quantitative analysis of TUNEL-positive myocardial cells in mice. Nuclei of normal cells are blue, and nuclei of apoptotic cells (TUNEL-positive cells) from the same fields are identified by green fluorescence (n = 5). *P < 0.05 versus WT mice treated with saline; #_P<_0.05 versus WT mice treated with ISO or AngII. Scale bar, 50 _μ_m. Original magnification, 400×.
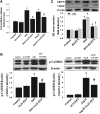
Fig. 4.
CYP2J2 overexpression restored CaMKII and SERCA2a expression and activity in mice failing hearts. (A) Elevated CaMKII activation induced by ISO or AngII was inhibited in CYP2J2 Tr mice (n = 5 per group). (B) Changes in b-IAM–SERCA2a and expression of SERCA2a were attenuated in CYP2J2 Tr mice exposed to ISO or AngII (n = 5 per group). (C) The activity of SERCA2a was restored in CYP2J2 Tr mice exposed to ISO or AngII (n = 5). (D) The detection of ROS using dihydroethidium (DHE) staining in mouse hearts. Quantification of ROS from three random fields per mouse (n = 5 per group). *P < 0.05 versus WT mice treated with saline; #_P<_0.05 versus WT mice treated with ISO or AngII. (E–G) The effect of Tempol on SERCA2a expression and activity, b-IAM–SERCA2a levels, and expression of ER stress markers (n = 5). *P < 0.05 versus control; #_P<_0.05 versus ISO. (H) Antioxidant enzyme expression in CYP2J2 Tr mice (n = 5 per group). *P < 0.05 versus WT mice treated with saline; #_P<_0.05 versus WT mice treated with ISO or AngII. Scale bar, 50 _μ_m. Original magnification, 400×.
Fig. 5.
14,15-EET reduced ER stress signaling and apoptosis in cultured cardiomyocytes. (A–D) Effects of 14,15-EET and/or 14,15-EEZE on ER stress signaling induced by TG (A), TM (B), ISO (C), and AngII (D). (E–H) Hoechst staining and Annexin V–fluorescein isothiocyanate (FITC) binding with flow cytometry analysis showed that 14,15-EET inhibited apoptosis induced by ISO or AngII, respectively (n = 3 for each experiment). *P < 0.05 versus control; #_P<0.05 versus ISO or AngII; ^_P < 0.05 versus ISO + EET or AngII + EET. DMSO, dimethyl sulfoxide.
Fig. 6.
14,15-EET attenuates the ER stress–induced increase of intracellular Ca2+ in cardiomyocytes. (A and B) 14,15-EET attenuated the rise in intracellular Ca2+ levels and overexpression of p-CaMKII in H9C2 cells exposed to ISO or AngII, respectively (n = 3 for each experiment). *P < 0.05 versus control; #_P<_0.05 versus ISO or AngII. (C) BAPTA attenuated the ER stress signaling in H9C2 cells exposed with ISO (n = 3 for each experiment). *P < 0.05 versus control; #_P<_0.05 versus ISO.
Fig. 7.
14,15-EET preserved the expression and activity of SERCA2a in cardiomyocytes after ER stress. (A) 14,15-EET attenuated the reduction of SERCA2a expression and prevented its oxidation in H9C2 cells treated with ISO or AngII, respectively. (B) 14,15-EET restored the activity of SERCA2a (n = 3 for each experiment). (C) 14,15-EET attenuated ISO- or AngII-induced ROS production in cardiomyocytes (n = 3 for each experiment). (D) 14,15-EET cotreatment attenuated the loss of ZnCu-SOD, Mn-SOD, and catalase protein levels observed in ISO- or AngII-treated cells (n = 3 for each experiment). *P < 0.05 versus control; #_P<_0.05 versus AngII.
Similar articles
- CYP2J2 and its metabolites (epoxyeicosatrienoic acids) attenuate cardiac hypertrophy by activating AMPKα2 and enhancing nuclear translocation of Akt1.
Wang B, Zeng H, Wen Z, Chen C, Wang DW. Wang B, et al. Aging Cell. 2016 Oct;15(5):940-52. doi: 10.1111/acel.12507. Epub 2016 Jul 14. Aging Cell. 2016. PMID: 27416746 Free PMC article. - Chronic inhibition of cGMP-specific phosphodiesterase 5 suppresses endoplasmic reticulum stress in heart failure.
Gong W, Duan Q, Cai Z, Chen C, Ni L, Yan M, Wang X, Cianflone K, Wang DW. Gong W, et al. Br J Pharmacol. 2013 Dec;170(7):1396-409. doi: 10.1111/bph.12346. Br J Pharmacol. 2013. PMID: 24032459 Free PMC article. - 14,15-epoxyeicosatrienoic Acid suppresses cigarette smoke extract-induced apoptosis in lung epithelial cells by inhibiting endoplasmic reticulum stress.
Yu G, Zeng X, Wang H, Hou Q, Tan C, Xu Q, Wang H. Yu G, et al. Cell Physiol Biochem. 2015;36(2):474-86. doi: 10.1159/000430113. Epub 2015 May 11. Cell Physiol Biochem. 2015. PMID: 25968975 - SERCA2a: a key protein in the Ca2+ cycle of the heart failure.
Zhihao L, Jingyu N, Lan L, Michael S, Rui G, Xiyun B, Xiaozhi L, Guanwei F. Zhihao L, et al. Heart Fail Rev. 2020 May;25(3):523-535. doi: 10.1007/s10741-019-09873-3. Heart Fail Rev. 2020. PMID: 31701344 Review. - The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases.
Kawase Y, Hajjar RJ. Kawase Y, et al. Nat Clin Pract Cardiovasc Med. 2008 Sep;5(9):554-65. doi: 10.1038/ncpcardio1301. Epub 2008 Jul 29. Nat Clin Pract Cardiovasc Med. 2008. PMID: 18665137 Review.
Cited by
- Antipurinergic therapy corrects the autism-like features in the Fragile X (Fmr1 knockout) mouse model.
Naviaux JC, Wang L, Li K, Bright AT, Alaynick WA, Williams KR, Powell SB, Naviaux RK. Naviaux JC, et al. Mol Autism. 2015 Jan 13;6:1. doi: 10.1186/2040-2392-6-1. eCollection 2015. Mol Autism. 2015. PMID: 25705365 Free PMC article. - Ophiopogonin D maintains Ca2+ homeostasis in rat cardiomyocytes in vitro by upregulating CYP2J3/EETs and suppressing ER stress.
You WT, Zhou T, Ma ZC, Liang QD, Xiao CR, Tang XL, Tan HL, Zhang BL, Wang YG, Gao Y. You WT, et al. Acta Pharmacol Sin. 2016 Mar;37(3):368-81. doi: 10.1038/aps.2015.146. Epub 2016 Feb 1. Acta Pharmacol Sin. 2016. PMID: 26838069 Free PMC article. - In vitro and in vivo metabolism of N-adamantyl substituted urea-based soluble epoxide hydrolase inhibitors.
Liu JY, Tsai HJ, Morisseau C, Lango J, Hwang SH, Watanabe T, Kim IH, Hammock BD. Liu JY, et al. Biochem Pharmacol. 2015 Dec 15;98(4):718-31. doi: 10.1016/j.bcp.2015.10.013. Epub 2015 Oct 19. Biochem Pharmacol. 2015. PMID: 26494425 Free PMC article. - The Role of Epoxyeicosatrienoic Acids in Cardiac Remodeling.
Lai J, Chen C. Lai J, et al. Front Physiol. 2021 Feb 24;12:642470. doi: 10.3389/fphys.2021.642470. eCollection 2021. Front Physiol. 2021. PMID: 33716791 Free PMC article. Review. - Ophiopogonin D Increases SERCA2a Interaction with Phospholamban by Promoting CYP2J3 Upregulation.
Wang J, You W, Wang N, Zhou W, Ge Y, Ma Z, Tan H, Wang Y, Gao Y. Wang J, et al. Oxid Med Cell Longev. 2020 Dec 31;2020:8857906. doi: 10.1155/2020/8857906. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33488937 Free PMC article.
References
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA. (2004) S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10:1200–1207 - PubMed
- Biagioli M, Pifferi S, Ragghianti M, Bucci S, Rizzuto R, Pinton P. (2008) Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium 43:184–195 - PubMed
- Cohen RA, Adachi T. (2006) Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc Med 16:109–114 - PubMed
- Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. (1999) Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res 84:210–219 - PubMed
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. (2008) Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27:285–299 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous