Ashwagandha (Withania somnifera) reverses β-amyloid1-42 induced toxicity in human neuronal cells: implications in HIV-associated neurocognitive disorders (HAND) - PubMed (original) (raw)
Ashwagandha (Withania somnifera) reverses β-amyloid1-42 induced toxicity in human neuronal cells: implications in HIV-associated neurocognitive disorders (HAND)
Kesava Rao Venkata Kurapati et al. PLoS One. 2013.
Abstract
Alzheimer's disease (AD) is characterized by progressive dysfunction of memory and higher cognitive functions with abnormal accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles throughout cortical and limbic brain regions. At present no curative treatment is available, and research focuses on drugs for slowing disease progression or providing prophylaxis. Withania somnifera (WS) also known as 'ashwagandha' is used widely in Ayurvedic medicine as a nerve tonic and memory enhancer. However, there is a paucity of data on the potential neuroprotective effects of W.somnifera against β-Amyloid (1-42)-induced neuropathogenesis. In the present study, we have tested the neuroprotective effects of methanol:Chloroform (3:1) extract of ashwagandha against β-amyloid induced toxicity and HIV-1Ba-L (clade B) infection using a human neuronal SK-N-MC cell line. Our results showed that β-amyloid induced cytotoxic effects in SK-N-MC cells as shown by decreased cell growth when tested individually. Also, confocal microscopic analysis showed decreased spine density, loss of spines and decreased dendrite diameter, total dendrite and spine area in clade B infected SK-N-MC cells compared to uninfected cells. However, when ashwagandha was added to β-amyloid treated and HIV-1 infected samples, the toxic effects were neutralized. Further, the MTT cell viability assays and the peroxisome proliferator-activated receptor-γ (PPARγ) levels supported these observations indicating the neuroprotective effect of WS root extract against β-amyloid and HIV-1Ba-L (clade B) induced neuro-pathogenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
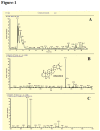
Figure 1. Compounds observed by LC-MS analysis of Methanol:Chloroform (3:1) extract of W.somnifera root.
A. HPLC profile showing the main components present. B. UV-Vis and mass spectra of the Withanolide A, identified as Withanolide A by comparison with a reference standard. C. UV-Vis and mass spectra of few other peaks. The structures of these components cannot be ascertained from these data alone and further studies are required.
Figure 2. Representative Giemsa stained flasks showing the effect of β-amyloid1-42, Ashwagandha and Ashwagandha plus β-amyloid1-42 on SK-N-MC cell line
1. Control, 2. β- Amyloid 1-42 treated, 3. Ashwagandha treated and 4. Ashwagandha plus β- Amyloid1-42 treated.
Figure 3. Representative microscopic observations of Sulforhodamine B (SRB) stained cultures showing the effect of β-amyloid1-42, Ashwagandha and Ashwagandha plus β-amyloid1-42 on SK-N-MC cell line (X80).
A. Control, B. β- Amyloid 1-42 treated, C. Ashwagandha treated and D. Ashwagandha plus β- Amyloid1-42 treated.
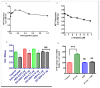
Figure 4. Modulatory effects of Ashwagandha and β- Amyloid1-42 on human neuronal cells.
A. Dose-response curve of Ashwagandha showing optimal concentrations and B. Dose-response cytotoxic effects of β-amyloid1-42 on SK-N-MC cells. Cells were treated with different concentrations of Ashwagandha / β-amyloid1-42 and mitochondrial function was determined by the MTT reduction assay as described in the Materials and Methods. C. MTT assay showing the inhibition of cell viability by β- Amyloid1-42 (βA) and its reversal by Ashwagandha (ASH) at different concentrations on SK-N-MC cell line D. Effect of β-amyloid1-42 on LDH leakage in SK-N-MC cells and its reversal by Ashwagandha. The data are expressed as mean ± SD of four independent experiments. (*) indicates a statistically significant difference compared to controls (p<0.05).
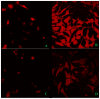
Figure 5. Congo red staining showing the increased internalization in β- Amyloid1-42 treated and its reversal by Ashwagandha in SK-N-MC cell line
A. Control, B. β- Amyloid 1-42 treated, C. Ashwagandha treated and D. Ashwagandha plus β- Amyloid1-42 treated. β- Amyloid1-42 cell internalization was observed by confocal laser microscopy: excitation 488- 543 nm and emission 560 nm; lens 20x / 0.5, 3 x. Images are from one representative experiment of two experiments performed.
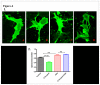
Figure 6. Confocal Images of DIL stained SK-N-MC cells showing the effect of β-amyloid1-42, Ashwagandha and Ashwagandha plus β-amyloid1-42.
1. A. Control, B. β- Amyloid 1-42 treated, C. Ashwagandha treated and D. Ashwagandha plus β- Amyloid1-42 treated. 2. Quantitative analysis showing the decreased spine density in β- Amyloid1-42 treated SK-N-MC cell line and its reversal by Ashwagandha (ASH). SK-N-MC cells were grown onto the glass coverslips, DIL stained and observed under confocal microscope. Randomly selected pictures in each group of the cells were captured in confocal microscope. Image J software was used to analyze the spine density, spine area, spine length and number of spines.
Figure 7. Confocal Images of DIL stained SK-N-MC cells showing the effect of HIV-1Ba-L (clade B), Ashwagandha and Ashwagandha plus HIV-1Ba-L (clade B).
1. A. Control, B. HIV-1Ba-L (clade B) treated, C. Ashwagandha treated and D. Ashwagandha plus HIV-1Ba-L (clade B) treated. 2. Quantitative analysis showing the decreased spine density in HIV-1Ba-L (clade B) treated SK-N-MC cell line and its reversal by Ashwagandha (ASH). SK-N-MC cells were grown onto the glass coverslips, DIL stained and observed under confocal microscope. Randomly selected pictures in each group of the cells were captured in confocal microscope. Image J software was used to analyze the spine density, spine area, spine length and number of spines.
Figure 8. Western blotting analysis showing the decreased PPARγ protein levels in β-amyloid treated and its reversal by Ashwagandha in SK-N-MC neuronal cells.
(A) Cell lysates were separated in 4% to 15% linear gradient SDS-PAGE gels and were probed against the respective antibodies. GAPDH was used as the loading control. (B) Quantitative analysis showing the decreased PPARγ protein levels in β- Amyloid1-42 treated cultures. ASH - Ashwagandha; β-amy - β-amyloid. The gel shown is a representative for three experiments.
Similar articles
- β-Amyloid1-42, HIV-1Ba-L (clade B) infection and drugs of abuse induced degeneration in human neuronal cells and protective effects of ashwagandha (Withania somnifera) and its constituent Withanolide A.
Kurapati KR, Samikkannu T, Atluri VS, Kaftanovskaya E, Yndart A, Nair MP. Kurapati KR, et al. PLoS One. 2014 Nov 21;9(11):e112818. doi: 10.1371/journal.pone.0112818. eCollection 2014. PLoS One. 2014. PMID: 25415340 Free PMC article. - Improving the inhibition of β-amyloid aggregation by withanolide and withanoside derivatives.
Dubey S, Kallubai M, Subramanyam R. Dubey S, et al. Int J Biol Macromol. 2021 Mar 15;173:56-65. doi: 10.1016/j.ijbiomac.2021.01.094. Epub 2021 Jan 16. Int J Biol Macromol. 2021. PMID: 33465364 - Neurodegenerative diseases and Withania somnifera (L.): An update.
Dar NJ, Muzamil Ahmad. Dar NJ, et al. J Ethnopharmacol. 2020 Jun 28;256:112769. doi: 10.1016/j.jep.2020.112769. Epub 2020 Mar 30. J Ethnopharmacol. 2020. PMID: 32240781 Review. - Withania somnifera (L.) Dunal - Modern perspectives of an ancient Rasayana from Ayurveda.
Mukherjee PK, Banerjee S, Biswas S, Das B, Kar A, Katiyar CK. Mukherjee PK, et al. J Ethnopharmacol. 2021 Jan 10;264:113157. doi: 10.1016/j.jep.2020.113157. Epub 2020 Aug 9. J Ethnopharmacol. 2021. PMID: 32783987 Review.
Cited by
- Acute and Repeated Ashwagandha Supplementation Improves Markers of Cognitive Function and Mood.
Leonard M, Dickerson B, Estes L, Gonzalez DE, Jenkins V, Johnson S, Xing D, Yoo C, Ko J, Purpura M, Jäger R, Faries M, Kephart W, Sowinski R, Rasmussen CJ, Kreider RB. Leonard M, et al. Nutrients. 2024 Jun 8;16(12):1813. doi: 10.3390/nu16121813. Nutrients. 2024. PMID: 38931168 Free PMC article. Clinical Trial. - Common gene-network signature of different neurological disorders and their potential implications to neuroAIDS.
Sagar V, Pilakka-Kanthikeel S, Martinez PC, Atluri VSR, Nair M. Sagar V, et al. PLoS One. 2017 Aug 8;12(8):e0181642. doi: 10.1371/journal.pone.0181642. eCollection 2017. PLoS One. 2017. PMID: 28792504 Free PMC article. - Arctigenin Treatment Protects against Brain Damage through an Anti-Inflammatory and Anti-Apoptotic Mechanism after Needle Insertion.
Song J, Li N, Xia Y, Gao Z, Zou SF, Kong L, Yao YJ, Jiao YN, Yan YH, Li SH, Tao ZY, Lian G, Yang JX, Kang TG. Song J, et al. Front Pharmacol. 2016 Jun 22;7:182. doi: 10.3389/fphar.2016.00182. eCollection 2016. Front Pharmacol. 2016. PMID: 27445818 Free PMC article. - Withania somnifera (L.) Dunal (Ashwagandha); current understanding and future prospect as a potential drug candidate.
Bhat JA, Akther T, Najar RA, Rasool F, Hamid A. Bhat JA, et al. Front Pharmacol. 2022 Dec 12;13:1029123. doi: 10.3389/fphar.2022.1029123. eCollection 2022. Front Pharmacol. 2022. PMID: 36578541 Free PMC article. Review. - β-Amyloid1-42, HIV-1Ba-L (clade B) infection and drugs of abuse induced degeneration in human neuronal cells and protective effects of ashwagandha (Withania somnifera) and its constituent Withanolide A.
Kurapati KR, Samikkannu T, Atluri VS, Kaftanovskaya E, Yndart A, Nair MP. Kurapati KR, et al. PLoS One. 2014 Nov 21;9(11):e112818. doi: 10.1371/journal.pone.0112818. eCollection 2014. PLoS One. 2014. PMID: 25415340 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH085259/MH/NIMH NIH HHS/United States
- 1R37DA025576/DA/NIDA NIH HHS/United States
- R01 DA021537/DA/NIDA NIH HHS/United States
- 1R01MH085259/MH/NIMH NIH HHS/United States
- 1R01DA027049/DA/NIDA NIH HHS/United States
- R01 DA027049/DA/NIDA NIH HHS/United States
- 5R01DA021537/DA/NIDA NIH HHS/United States
- R37 DA025576/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources