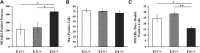Human APOE genotype affects intraneuronal Aβ1-42 accumulation in a lentiviral gene transfer model - PubMed (original) (raw)
. 2014 Mar 1;23(5):1365-75.
doi: 10.1093/hmg/ddt525. Epub 2013 Oct 23.
Affiliations
- PMID: 24154541
- PMCID: PMC3919009
- DOI: 10.1093/hmg/ddt525
Human APOE genotype affects intraneuronal Aβ1-42 accumulation in a lentiviral gene transfer model
Wenjuan Zhao et al. Hum Mol Genet. 2014.
Abstract
Intraneuronal accumulation of β-amyloid (Aβ)42 is one of the earliest pathological events in humans and in animal models of Alzheimer's disease (AD). Apolipoprotein E 4 (APOE4) is the major identified genetic risk factor for late-onset AD, with Aβ deposition beginning earlier in apoE4-positive subjects. To directly determine the effects of APOE genotype on intraneuronal accumulation of Aβ1-42 at the onset of AD pathogenesis, we introduced lentiviral Aβ1-42 into the cortex of APOE targeted replacement (TR) mice at the age of 8-9 months. We demonstrated a significant isoform-dependent effect of human APOE, with dramatically enhanced intracellular Aβ1-42 deposits in the cerebral cortex of APOE4-TR mice 2 weeks after injection. Double-immunofluorescent staining showed that intracellular accumulation of lentiviral Aβ1-42 was mainly present in neurons, localized to late endosomes/lysosomes. This intraneuronal accumulation of Aβ1-42 correlated with increased tau phosphorylation and cell death in the ipsilateral cortex around the injection site. Aβ1-42 was also observed in microglia, but not in astrocytes. Quantitative analysis revealed more neurons with Aβ1-42 while less microglia with Aβ1-42 nearest to the injection site of Aβ1-42 lentivirus in APOE4-TR mice. Finally, apoE was present in neurons of the ipsilateral cortex of APOE-TR mice at 2 weeks after lentivirus injection, in addition to astrocytes and microglia in both the ipsilateral and contralateral cerebral cortex. Taken together, these results demonstrate that apoE4 tips the balance of the glial and neuronal Aβ toward the intraneuronal accumulation of Aβ.
Figures
Figure 1.
Microinjection and intraneuronal expression of lentiviral Aβ1–42. (A) The schematic of the microinjection site of lentiviral Aβ1–42 and its distribution in the mouse cortex. The stereotaxic coordinates for the primary motor cortex of mouse were 1.6 mm lateral (left), 1.6 mm ventral and 0.5 mm anterior. Aβ1–42 viral stocks (6 μl) were injected through a microsyringe pump controller at a rate of 0.2 μl/min. (B and C) showed MOAB2 immunofluorescent staining in the contralateral (right) cortical area of a C57BL/6J mouse brain section without expression of lentiviral Aβ1–42 at magnifications of 20× and 40×. (D–F) showed Representative images of MOAB2 immunofluorescent staining in ipsilateral (left) cortex near the injection area of the mouse brain section at magnifications of 20×, 40× and 100×, respectively. In the higher magnifications, the staining of lentiviral Aβ1–42 was intracellular with a punctate pattern. Scale bars: B, D, 50 μm; C, E, 20 μm; F, 10 μm.
Figure 2.
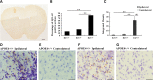
Intracellular accumulation of lentiviral Aβ1–42 is greatest in APOE4-TR mice at 2 weeks after lentivirus injection. (A) Representative image of a coronal brain section with DAB staining of biotinylated MOAB2 for lentiviral Aβ1–42 from APOE4-TR mouse. Scale bar = 500 μm. (B) The percentage of MOAB2-positive sections was significantly higher in APOE4-TR mice than that in APOE2- or APOE3-TR mice (**P < 0.01, APOE4+/+ versus APOE2+/+, APOE4+/+ versus APOE3+/+, Fisher's exact test). (C) The mean integrated density of MOAB2-positive staining in the ipsilateral cortex of APOE4-TR mice was significantly higher than that of APOE2- or APOE3-TR mice. The intensity of DAB stained images at 10× magnification was quantified by using the Image J software. Data are expressed as mean ± SEM and were analyzed by two-way ANOVA followed by Tukey's post hoc analyses. **P < 0.01, ipsilateral cortex of APOE4+/+ versus ipsilateral cortex of APOE2+/+ and APOE3+/+, ##P < 0.01, ipsilateral cortex of APOE4+/+ versus contralateral cortex of APOE4+/+. (D) Combination of DAB-labeling immunohistochemistry for MOAB2 (brown) with Nissl staining (blue) in the ipsilateral cortex near the injection site of Aβ1–42 lentivirus on a brain section of APOE4-TR mouse. Arrows and arrowheads indicate MOAB2 immunopositive staining in neuron- and glia-like cells, respectively. (E) Combination of DAB staining for MOAB2 (brown) with Nissl staining (blue) in the contralateral cortex on a brain section of APOE4-TR mouse. (F) Representative images of DAB staining for phospho-tau pSer199/202 antibody (AT8, brown) with Nissl staining (blue) in the ipsilateral cortex near the injection site of Aβ1–42 lentivirus on a brain section of APOE4-TR mouse. Arrows indicate phosphorylated tau in neurons. Solid arrowheads indicate phosphorylated tau in the absence of Nissl staining. Blank arrowheads indicate phosphorylated tau in neuronal processes. (G) Combination of DAB staining for AT8 antibody (brown) with Nissl staining (blue) in the contralateral cortex on a brain section of APOE4-TR mouse. Scale bars = 50 μm.
Figure 3.
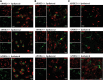
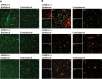
Lentiviral Aβ1–42 is mainly present in neurons, to a lesser extent in microglia, but not in astrocytes at 2 weeks after injection of lentivirus into the brain cortex of APOE2-, APOE3- and APOE4-TR mice. (A) Aβ1–42 (MOAB2, red) mainly deposited in cortical neurons (NeuN, green), displayed a punctate perinuclear localization. (B) Aβ1–42 (MOAB2, red) was also present in some microglia (Iba1, green). (C) Aβ1–42 (MOAB2, red) was not observed in astrocytes (GFAP, green). Scale bar = 10 μm.
Figure 4.
APOE genotype affects Aβ1–42 accumulation in neurons and glia. (A) Average number of MOAB2-positive neurons per mouse in the ipsilateral cortex near the injection site of Aβ1–42 lentivirus. (B) The average numbers of microglia per mouse were determined from Iba1 stains. (C) MOAB2-positive microglia (MOAB2-/Iba1-double-positive cells) in the ipsilateral cortex near the injection site of Aβ1–42 lentivirus were determined per mouse. Data were expressed as mean ± SEM and were analyzed by one-way ANOVA followed by Tukey's post hoc analyses. *P < 0.05, **P < 0.01.
Figure 5.
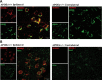
Intraneuronal accumulation of lentiviral Aβ1–42 is mainly localized to late endosomes/lysosomes, not early endosomes, in the ipsilateral cortex at 2 weeks after lentiviral injection. (A) Intraneuronal Aβ1–42 detected with MOAB2 (red) co-localized with late endosomal/lysosomal marker ABL-93 (anti-LAMP-2, green) in the ipsilateral cortex of an APOE4-TR mouse. (B) Intraneuronal Aβ1–42 (MOAB2, red) did not obviously co-localize with early endosomal marker EEA1 (green) in the ipsilateral cortex of an APOE4-TR mouse. Scale bar = 10 μm. Colocalization of antibodies appears as yellow.
Figure 6.
Immunohistochemistry of apoE in the brain cortex of APOE-TR mice at 2 weeks after injection of lentiviral Aβ1–42. (A) ApoE-positive cells (green) in both the ipsilateral and contralateral brain cortex. (B) In the ipsilateral cerebral cortex, apoE (green) is present in Aβ1–42-positive neurons (MOAB2, red), as well as microglia (Iba1, red) and astrocytes (GFAP, red). In the contralateral cerebral cortex, apoE is mainly present in microglia and astrocytes. Scale bar = 20 μm.
Similar articles
- Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition.
Rodriguez GA, Tai LM, LaDu MJ, Rebeck GW. Rodriguez GA, et al. J Neuroinflammation. 2014 Jun 19;11:111. doi: 10.1186/1742-2094-11-111. J Neuroinflammation. 2014. PMID: 24948358 Free PMC article. - Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Aβ metabolism in apoE4-targeted replacement mice.
Hu J, Liu CC, Chen XF, Zhang YW, Xu H, Bu G. Hu J, et al. Mol Neurodegener. 2015 Mar 5;10:6. doi: 10.1186/s13024-015-0001-3. Mol Neurodegener. 2015. PMID: 25871773 Free PMC article. - Accumulation of intraneuronal Abeta correlates with ApoE4 genotype.
Christensen DZ, Schneider-Axmann T, Lucassen PJ, Bayer TA, Wirths O. Christensen DZ, et al. Acta Neuropathol. 2010 May;119(5):555-66. doi: 10.1007/s00401-010-0666-1. Epub 2010 Mar 10. Acta Neuropathol. 2010. PMID: 20217101 Free PMC article. - ApoE4 reduction: An emerging and promising therapeutic strategy for Alzheimer's disease.
Li Y, Macyczko JR, Liu CC, Bu G. Li Y, et al. Neurobiol Aging. 2022 Jul;115:20-28. doi: 10.1016/j.neurobiolaging.2022.03.011. Epub 2022 Mar 22. Neurobiol Aging. 2022. PMID: 35453035 Free PMC article. Review. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
- Analyzing dendritic spine pathology in Alzheimer's disease: problems and opportunities.
Dorostkar MM, Zou C, Blazquez-Llorca L, Herms J. Dorostkar MM, et al. Acta Neuropathol. 2015 Jul;130(1):1-19. doi: 10.1007/s00401-015-1449-5. Epub 2015 Jun 11. Acta Neuropathol. 2015. PMID: 26063233 Free PMC article. Review. - Apolipoprotein E intersects with amyloid-β within neurons.
Konings SC, Nyberg E, Martinsson I, Torres-Garcia L, Klementieva O, Guimas Almeida C, Gouras GK. Konings SC, et al. Life Sci Alliance. 2023 Jun 8;6(8):e202201887. doi: 10.26508/lsa.202201887. Print 2023 Aug. Life Sci Alliance. 2023. PMID: 37290814 Free PMC article. - Altered Cholesterol Intracellular Trafficking and the Development of Pathological Hallmarks of Sporadic AD.
Chen X, Hui L, Soliman ML, Geiger JD. Chen X, et al. J Parkinsons Dis Alzheimers Dis. 2014;1(1):8. doi: 10.13188/2376-922x.1000002. J Parkinsons Dis Alzheimers Dis. 2014. PMID: 25621310 Free PMC article. - More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration.
Lane-Donovan C, Philips GT, Herz J. Lane-Donovan C, et al. Neuron. 2014 Aug 20;83(4):771-87. doi: 10.1016/j.neuron.2014.08.005. Neuron. 2014. PMID: 25144875 Free PMC article. Review. - Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3.
Guo YJ, Dong SY, Cui XX, Feng Y, Liu T, Yin M, Kuo SH, Tan EK, Zhao WJ, Wu YC. Guo YJ, et al. Mol Nutr Food Res. 2016 Oct;60(10):2161-2175. doi: 10.1002/mnfr.201600111. Epub 2016 Jul 18. Mol Nutr Food Res. 2016. PMID: 27296520 Free PMC article.
References
- LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous