Reduced Alzheimer's disease pathology by St. John's Wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice - PubMed (original) (raw)
Reduced Alzheimer's disease pathology by St. John's Wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice
Jacqueline Hofrichter et al. Curr Alzheimer Res. 2013 Dec.
Abstract
Soluble β-amyloid peptides (Aβ) and small Aβ oligomers represent the most toxic peptide moieties recognized in brains affected by Alzheimer's disease (AD). Here we provide the first evidence that specific St. John's wort (SJW) extracts both attenuate Aβ-induced histopathology and alleviate memory impairments in APP-transgenic mice. Importantly, these effects are attained independently of hyperforin. Specifically, two extracts characterized by low hyperforin content (i) significantly decrease intracerebral Aβ42 levels, (ii) decrease the number and size of amyloid plaques, (iii) rescue neocortical neurons, (iv) restore cognition to normal levels, and (iv) activate microglia in vitro and in vivo. Mechanistically, we reveal that the reduction of soluble Aβ42 species is the consequence of a highly increased export activity in the bloodbrain barrier ABCC1transporter, which was found to play a fundamental role in Aβ excretion into the bloodstream. These data (i) support the significant beneficial potential of SJW extracts on AD proteopathy, and (ii) demonstrate for the first time that hyperforin concentration does not necessarily correlate with their therapeutic effects. Hence, by activating ABC transporters, specific extracts of SJW may be used to treat AD and other diseases involving peptide accumulation and cognition impairment. We propose that the anti-depressant and anti-dementia effects of these hyperforin-reduced phytoextracts could be combined for treatment of the elderly, with a concomitant reduction in deleterious hyperforin-related side effects.
Figures
Figure 1
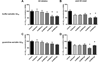
80%-ethanol St. John’s wort (SJW) extracts reduce brain Aβ42 levels independently of the hyperforin content. (A, B) Both SJW80 extracts reduced buffer-soluble Aβ42 significantly by −39% (SJW80low) and −38% (SJW80high) in the ‘AD initiation’ paradigm, and by −53% (SJW80low) and −49% (SJW80high) in the ‘post AD onset’ treatment paradigm, respectively. (C, D) Guanidine-soluble Aβ42 fraction characterized by insoluble oligomers and small fibril aggregates were significantly reduced only in the post-onset treatment. Here, the SJW80low extract showed a reduction by −50% and was even more potent than the SJW80high extract with a reduction by −31% (mean + SEM, *p≤0.05).
Figure 2
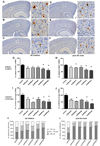
80%-ethanol SJW extracts reduced Aβ plaque number and size. (A-F) Histological presentation of plaque number and size reduction: (A) vehicle treatment, (B) SJWwater, (C) SJW60low, (D) SJW60high, (E) SJW80low, (F) SJW80high (scale bars: 500 µm, 50 µm). (G) 80%-ethanol extracts elicited the strongest reduction of plaque number by −29% (SJW80low) and −40% (SJW80high) in the ‘AD initiation’ group; and −36% (SJW80low) and −39% (SJW80high) in the ‘post AD onset’ treatment group. (H) Due to a strong plaque size reduction, the cortical coverage by plaques was finally significantly decreased by −47% (SJW80low) and −51% (SJW80high) after AD initiation and −52% (SJW80low) and −60% (SJW80high) after post-onset treatment, independently of the hyperforin content. (K, L) SJW treatment reduced plaque growth resulting in a significant size shift from large (>700µm2), via medium (400–700 µm2) to small plaques (<400 µm2). All SJW extracts significantly reduced the development of large plaques in the ‘post AD onset’ treatment paradigm (mean + SEM, *p≤0.05)
Figure 3
80%-ethanol SJW extract treatment protects against spatial memory impairment and neuronal loss. (A, B) Immunhistochemical NeuN labelings (neurons) of the neuronal layers I-V in the neocortex: (A) vehicle treatment, (B) SJW80low treatment (scale bars: 50 µm). (C, D) Quantification reveals strong neuroprotective effects in the ‘AD initiation’ group (+24% SJW80low, +21% SJW80high), and the ‘post AD onset’ treatment group (+17% SJW80low, +22% SJW80high) for both 80%-ethanol SJW extracts. The recovery of the NeuN-labeled area was even similar to levels in non-transgenic controls. (E, F) Spatial memory improvement was evaluated by Morris water maze. 80%-ethanol SJW extracts protect from cognitive decline as shown by the reduced escape latency by −50% (SJW80low) and −53% (SJW80high) in the ‘AD initiation’ group (E, all days are shown) and by −41% (SJW80low) and −46% (SJW80high) in the ‘post AD onset’ treatment group (F, day 4 shown). Memory performance was comparable to non-transgenic controls (mean + SEM, *p≤0.05).
Figure 4
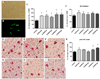
80%-ethanol SJW extracts enhance anti-Aβ activity of microglia in vivo and in vitro. (A-C) In vitro treatment of a primary microglia cell culture with SJW extracts and FITC-labeled Aβ42 (A, bright-field; B, fluorescence micrographs) revealed activating effects by crude SJW extracts independent of hyperforin content. Quantification of phagocytized Aβ42 by microglia resulted in enhanced fluorescence: +60% SJWwater, +63% SJW60low, +46% SJW60high, +78% SJW80low, +78% SJW80high. (D-I) Microphotographs of cortical microglia in 100-days-old mice with ‘post AD onset’ treatment: (D) vehicle, (E) SJWwater, (F) SJW60low, (G) SJW60high, (H) SJW80low, (I) SJW80high indicated stimulating properties of SJW (scale bars: 50 µm). (J; D) Quantification of microglial area in the vicinity of Aβ-plaques revealed an activation of microglia with SJW80 in the ‘post AD onset’ treatment group by +23% (SJW80low) and +42% (SJW80high) independently of the hyperforin content (mean + SEM, *p≤0.05).
Figure 5
SJW extracts do not affect BACE1 and ADAM10 expression, as shown by Western blot quantification of neocortical homogenates of 100-day-old female APP-tg mice. Neither ’AD initiation’ nor ’post AD onset’ treatment affected expression as compared to vehicle-treated littermates.
Figure 6
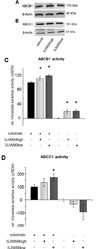
80%-ethanol, low hyperforin SJW extracts activate the Aβ exporter ABCC1. Western blot analyses of neocortical homogenates revealed no changes in the expression of (A) ABCB1, nor (B) ABCC1 after treatment with high or low hyperforin containing extracts. (C) SJW extracts enhance ABCB1 transporter activity (substrate: verapamil), but the elevated activity is consumed by the active extract substance itself as indicated by activity elevation in the absence of verapamil. (D) Only the SJW80low extract significantly enhances ABCC1 transporter activity (substrate: NEM-GS) independently of the hyperforin content without being an ABCC1 substrate itself (mean ± SEM, *p≤0.05).
Similar articles
- Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-beta-deposits.
Dinamarca MC, Cerpa W, Garrido J, Hancke JL, Inestrosa NC. Dinamarca MC, et al. Mol Psychiatry. 2006 Nov;11(11):1032-48. doi: 10.1038/sj.mp.4001866. Epub 2006 Jul 25. Mol Psychiatry. 2006. PMID: 16880827 - St. John's Wort reduces beta-amyloid accumulation in a double transgenic Alzheimer's disease mouse model-role of P-glycoprotein.
Brenn A, Grube M, Jedlitschky G, Fischer A, Strohmeier B, Eiden M, Keller M, Groschup MH, Vogelgesang S. Brenn A, et al. Brain Pathol. 2014 Jan;24(1):18-24. doi: 10.1111/bpa.12069. Epub 2013 Jun 28. Brain Pathol. 2014. PMID: 23701205 Free PMC article. - Neurobiological effects of Hyperforin and its potential in Alzheimer's disease therapy.
Griffith TN, Varela-Nallar L, Dinamarca MC, Inestrosa NC. Griffith TN, et al. Curr Med Chem. 2010;17(5):391-406. doi: 10.2174/092986710790226156. Curr Med Chem. 2010. PMID: 20015041 Review. - The hyperforin derivative IDN5706 occludes spatial memory impairments and neuropathological changes in a double transgenic Alzheimer's mouse model.
Cerpa W, Hancke JL, Morazzoni P, Bombardelli E, Riva A, Marin PP, Inestrosa NC. Cerpa W, et al. Curr Alzheimer Res. 2010 Mar;7(2):126-33. doi: 10.2174/156720510790691218. Curr Alzheimer Res. 2010. PMID: 19939230 - Understanding drug interactions with St John's wort (Hypericum perforatum L.): impact of hyperforin content.
Chrubasik-Hausmann S, Vlachojannis J, McLachlan AJ. Chrubasik-Hausmann S, et al. J Pharm Pharmacol. 2019 Jan;71(1):129-138. doi: 10.1111/jphp.12858. Epub 2018 Feb 7. J Pharm Pharmacol. 2019. PMID: 29411879 Review.
Cited by
- Apolar Extracts of St. John's Wort Alleviate the Effects of β-Amyloid Toxicity in Early Alzheimer's Disease.
El Menuawy A, Brüning T, Eiriz I, Hähnel U, Marthe F, Möhle L, Górska AM, Santos-García I, Wangensteen H, Wu J, Pahnke J. El Menuawy A, et al. Int J Mol Sci. 2024 Jan 21;25(2):1301. doi: 10.3390/ijms25021301. Int J Mol Sci. 2024. PMID: 38279301 Free PMC article. - Unprecedented polycyclic polyprenylated acylphloroglucinols with anti-Alzheimer's activity from St. John's wort.
Guo Y, Huang F, Sun W, Zhou Y, Chen C, Qi C, Yang J, Li XN, Luo Z, Zhu H, Wang X, Zhang Y. Guo Y, et al. Chem Sci. 2021 Jul 26;12(34):11438-11446. doi: 10.1039/d1sc03356e. eCollection 2021 Sep 1. Chem Sci. 2021. PMID: 34567498 Free PMC article. - Measurement of cerebral ABCC1 transport activity in wild-type and APP/PS1-21 mice with positron emission tomography.
Zoufal V, Mairinger S, Krohn M, Wanek T, Filip T, Sauberer M, Stanek J, Kuntner C, Pahnke J, Langer O. Zoufal V, et al. J Cereb Blood Flow Metab. 2020 May;40(5):954-965. doi: 10.1177/0271678X19854541. Epub 2019 Jun 13. J Cereb Blood Flow Metab. 2020. PMID: 31195936 Free PMC article. - Enhancing of cerebral Abeta clearance by modulation of ABC transporter expression: a review of experimental approaches.
Loeffler DA. Loeffler DA. Front Aging Neurosci. 2024 May 30;16:1368200. doi: 10.3389/fnagi.2024.1368200. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38872626 Free PMC article. Review. - Unified theory of Alzheimer's disease (UTAD): implications for prevention and curative therapy.
Nehls M. Nehls M. J Mol Psychiatry. 2016 Jul 15;4:3. doi: 10.1186/s40303-016-0018-8. eCollection 2016. J Mol Psychiatry. 2016. PMID: 27429752 Free PMC article. Review.
References
- Alloul K, Sauriol L, Kennedy W, Laurier C, Tessier G, Novosel S, et al. Alzheimer's disease: a review of the disease, its epidemiology and economic impact. Arch Gerontol Geriatr. 1998;27(3):189–221. - PubMed
- Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol Rev. 2002;54(3):469–525. - PubMed
- Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993;32(18):4693–4697. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical