Age-dependent dysregulation of innate immunity - PubMed (original) (raw)
Review
. 2013 Dec;13(12):875-87.
doi: 10.1038/nri3547. Epub 2013 Oct 25.
Affiliations
- PMID: 24157572
- PMCID: PMC4096436
- DOI: 10.1038/nri3547
Review
Age-dependent dysregulation of innate immunity
Albert C Shaw et al. Nat Rev Immunol. 2013 Dec.
Abstract
As we age, the innate immune system becomes dysregulated and is characterized by persistent inflammatory responses that involve multiple immune and non-immune cell types and that vary depending on the cell activation state and tissue context. This ageing-associated basal inflammation, particularly in humans, is thought to be induced by several factors, including the reactivation of latent viral infections and the release of endogenous damage-associated ligands of pattern recognition receptors (PRRs). Innate immune cell functions that are required to respond to pathogens or vaccines, such as cell migration and PRR signalling, are also impaired in aged individuals. This immune dysregulation may affect conditions associated with chronic inflammation, such as atherosclerosis and Alzheimer's disease.
Figures
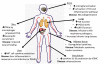
Figure 1. Organ-specific changes of the innate immune system associated with ageing and disease
The effects of aging in various organ systems are depicted. Diminished innate immune responses are found in some cases—such as in skin, where a decrease in TNF production by macrophages results in decreased endothelial cell activation and diminished DTH responses. In addition, studies in mice have linked diminished neutrophil recruitment to impaired wound healing. Decreased NK cell function and induced NLRP3 function in response to influenza virus influence infection in the lung. However, there are also examples of dysregulated inflammatory responses found in aging. For example, in the lung following burn injury in a murine model, there is increased neutrophil inflammation. In the liver there is experimental data showing that NKT cells exhibit exaggerated inflammatory responses that contribute to immune pathology during viral infection or to TLR activators. In the brain, TLR and inflammasome signalling are elevated with aging, and are associated with microglial activation. Signalling via the NLRP3 inflammasome contributes to increased age-associated inflammation in adipose tissue as well. Finally, within the vasculature, increased basal IL-6 production by vascular smooth muscle cells has been found in aged rodents and non-human primates and may provide a potential explanation for the increased prevalence of atherosclerosis with aging.
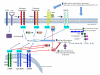
Figure 2. Effects of ageing on innate immune PRR signalling
TLR and NLRP3 signalling pathways are depicted, with NF-κB-dependent pathways indicated in blue and type I interferon pathways indicated in red. With aging, elevated levels of PRR ligands (DAMPs) arising from cell damage, necrosis, lipotoxicity or other causes contribute to an elevated pro-inflammatory state, as manifested by increased basal levels of proinflammatory cytokines that may result in part from both TLR and NLRP3 signalling. Such basal activation may restrict responsiveness to new pathogens or vaccines, resulting in innate immune failure and impaired adaptive immune responses. Changes in protein expression for TLR1 and TLR5 in humans are indicated; results in mice suggest either a widespread decrease in TLR gene expression, or preserved expression with changes in intracellular signaling proteins (e.g. p38 MAP kinase). Abbreviations: TLR—Toll-like Receptor; IFNAR—type I Interferon receptor; MyD88—myeloid differentiation primary response 88; TRIF—Toll/IL1 receptor domain-containing adapter-inducing interferon-β; TRAM—TRIF-related adaptor molecule; IRF3—interferon regulatory factor 3; IRF7—interferon regulatory factor 7; NLRP3—NOD-like receptor family, pyrin domain containing 3; ASC—Apoptosis-associated speck-like protein containing a CARD.
Similar articles
- Innate immune perturbations, accumulating DAMPs and inflammasome dysregulation: A ticking time bomb in ageing.
Kapetanovic R, Bokil NJ, Sweet MJ. Kapetanovic R, et al. Ageing Res Rev. 2015 Nov;24(Pt A):40-53. doi: 10.1016/j.arr.2015.02.005. Epub 2015 Feb 25. Ageing Res Rev. 2015. PMID: 25725308 Review. - Innate recognition of microbial-derived signals in immunity and inflammation.
Zhang Y, Liang C. Zhang Y, et al. Sci China Life Sci. 2016 Dec;59(12):1210-1217. doi: 10.1007/s11427-016-0325-6. Epub 2016 Nov 23. Sci China Life Sci. 2016. PMID: 27888386 Review. - Host Resistance and Immune Aging.
Bandaranayake T, Shaw AC. Bandaranayake T, et al. Clin Geriatr Med. 2016 Aug;32(3):415-32. doi: 10.1016/j.cger.2016.02.007. Epub 2016 May 25. Clin Geriatr Med. 2016. PMID: 27394014 Free PMC article. Review. - Guardians of the Cell: Effector-Triggered Immunity Steers Mammalian Immune Defense.
Kufer TA, Creagh EM, Bryant CE. Kufer TA, et al. Trends Immunol. 2019 Oct;40(10):939-951. doi: 10.1016/j.it.2019.08.001. Epub 2019 Sep 6. Trends Immunol. 2019. PMID: 31500957 Review. - Identification and functions of pattern-recognition receptors.
Kumagai Y, Akira S. Kumagai Y, et al. J Allergy Clin Immunol. 2010 May;125(5):985-92. doi: 10.1016/j.jaci.2010.01.058. Epub 2010 Apr 14. J Allergy Clin Immunol. 2010. PMID: 20392481 Review.
Cited by
- The 3 I's of immunity and aging: immunosenescence, inflammaging, and immune resilience.
Wrona MV, Ghosh R, Coll K, Chun C, Yousefzadeh MJ. Wrona MV, et al. Front Aging. 2024 Oct 16;5:1490302. doi: 10.3389/fragi.2024.1490302. eCollection 2024. Front Aging. 2024. PMID: 39478807 Free PMC article. Review. - Probing the Interface of HIV and Inflammaging.
Sieg SF, Shive CL, Panigrahi S, Freeman ML. Sieg SF, et al. Curr HIV/AIDS Rep. 2021 Jun;18(3):198-210. doi: 10.1007/s11904-021-00547-0. Epub 2021 Mar 11. Curr HIV/AIDS Rep. 2021. PMID: 33709322 Review. - Activation of TLR7 and Innate Immunity as an Efficient Method Against COVID-19 Pandemic: Imiquimod as a Potential Therapy.
Poulas K, Farsalinos K, Zanidis C. Poulas K, et al. Front Immunol. 2020 Jun 11;11:1373. doi: 10.3389/fimmu.2020.01373. eCollection 2020. Front Immunol. 2020. PMID: 32612613 Free PMC article. No abstract available. - Immune Response, Inflammation, and the Clinical Spectrum of COVID-19.
García LF. García LF. Front Immunol. 2020 Jun 16;11:1441. doi: 10.3389/fimmu.2020.01441. eCollection 2020. Front Immunol. 2020. PMID: 32612615 Free PMC article. Review. - Coronavirus Disease 2019 (COVID-19) and Nutritional Status: The Missing Link?
Silverio R, Gonçalves DC, Andrade MF, Seelaender M. Silverio R, et al. Adv Nutr. 2021 Jun 1;12(3):682-692. doi: 10.1093/advances/nmaa125. Adv Nutr. 2021. PMID: 32975565 Free PMC article. Review.
References
- Population Division. Department of Economic and Social Affairs. United Nations . World Population Ageing: 1950-2050. United Nations; New York: 2001.
- Centers for Disease Control and Prevention . National Ambulatory Medical Survey. National Center for Health Statistics; 2005.
- Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–933. - PubMed
- Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age--age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31:137–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN272201100019C/AI/NIAID NIH HHS/United States
- AG033049/AG/NIA NIH HHS/United States
- AG042489/AG/NIA NIH HHS/United States
- N01272201100019C-3-0-1/PHS HHS/United States
- U19 AI089992/AI/NIAID NIH HHS/United States
- AG028082/AG/NIA NIH HHS/United States
- K24 AG042489/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials