Proangiogenic TIE2(+)/CD31 (+) macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes - PubMed (original) (raw)
doi: 10.1007/s10059-013-0194-7. Epub 2013 Oct 24.
Gun-Hyung Kang, Hyungjoon Noh, Ji-Young Cha, Ho-Jae Lee, Jeong-Hwan Yoon, Mizuko Mamura, Jeong-Seok Nam, Dae Ho Lee, Young A Kim, Young Joo Park, Hyeonjin Kim, Byung-Chul Oh
Affiliations
- PMID: 24158612
- PMCID: PMC3887941
- DOI: 10.1007/s10059-013-0194-7
Proangiogenic TIE2(+)/CD31 (+) macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes
Ok-Hee Kim et al. Mol Cells. 2013 Nov.
Erratum in
- Mol Cells. 2014 Feb;37(2):188
Abstract
Tumor-associated macrophages (TAMs) accumulate in various cancers and promote tumor angiogenesis and metastasis, and thus may be ideal targets for the clinical diagnosis of tumor metastasis with high specificity. However, there are few specific markers to distinguish between TAMs and normal or inflammatory macrophages. Here, we show that TAMs localize in green fluorescent protein-labeled tumors of metastatic lymph nodes (MLNs) from B16F1 melanoma cells but not in necrotic tumor regions, suggesting that TAMs may promote the growth of tumor cells and the progression of tumor metastasis. Furthermore, we isolated pure populations of TAMs from MLNs and characterized their gene expression signatures compared to peritoneal macrophages (PMs), and found that TAMs significantly overexpress immunosuppressive cytokines such as IL-4, IL-10, and TGF-β as well as proangiogenic factors such as VEGF, TIE2, and CD31. Notably, immunological analysis revealed that TIE2(+)/CD31(+) macrophages constitute the predominant population of TAMs that infiltrate MLNs, distinct from tissue or inflammatory macrophages. Importantly, these TIE2(+)/CD31(+) macrophages also heavily infiltrated MLNs from human breast cancer biopsies but not reactive hyperplastic LNs. Thus, TIE2(+)/ CD31(+) macrophages may be a unique histopathological biomarker for detecting metastasis in clinical diagnosis, and a novel and promising target for TAM-specific cancer therapy.
Figures
Fig. 1
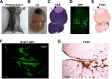
Histopathological analysis of a metastatic lymph nodes from a B16F1-GFP melanoma mouse. (A) Representative B16F1-GFP melanoma mouse with a primary tumor. (B-E) Histological analyses of a representative metastatic lymph node section: gross photograph (B), H&E staining (C), GFP fluorescence (D), and IHC staining with the F4/80 antibody (E). Scale bars, 500 μm. The arrow indicates necrotic regions of the tumor. (F-G) Magnified views of fluorescence images of the rectangular regions (F) and IHC staining of the same magnified rectangular regions (G). Scale bars, 100 μm.
Fig. 2
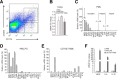
Isolation of CD11b+ TAMs from metastatic lymph nodes and comparative expression profiles of 18 genes associated with the macrophage phenotype for PMs, LPS-stimulated PMs (PM-LPS), and CD11b+ TAMs. (A) TAMs were isolated from metastatic lymph nodes of 24 B16F1 melanoma mice each time, and stained with anti-CD11b and anti-CD206 antibodies before sorting by FACS. (B) mRNA expression of TGF-β in PMs, PM-LPS, and FACS-sorted CD11b+ TAMs from metastatic lymph nodes. Data are presented as means ± SE for each group (n = 3). *P < 0.05, compared to PMs. (C-E) qRT-PCR analysis of gene expression in PMs (C), PM-LPS (D), and FACS-sorted CD11b+ TAMs (E) from metastatic lymph nodes (_n_ = 24). All values are expressed relative to cyclophilin A and adjusted arithmetically to depict the TGF-β expression for each macro-Phage as 100 units. Values represent the means and ± SE of three independent samples for each macrophage. Arbitrary cut-offs were set at CT < 25 (abundant), 25 < CT < 30 (present), and CT > 30 (absent), as shown by broken lines in PM. For VEGF, IL-4, and IL-10, all values are expressed as mean fold-change relative to PMs. (F) mRNA expression in PMs, PM-LPS, and FACS-sorted CD11b+ TAMs from metastatic lymph nodes. Data are presented as means ± SE for each group (n = 3). *P < 0.05, compared to PMs.
Fig. 3
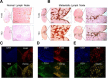
Immunological assessment of TIE2 and CD31 protein as TAM-specific markers of micro- or macro-metastasis. (A) IHC staining of serial sections from normal lymph nodes using anti-F4/80 and anti-TIE2 antibodies. Boxed regions (left) are magnified views of the right panel. Scale bars, 100 μm. (B) IHC staining of micro-meta-static lymph nodes (left) and macro-metastatic lymph nodes (right) using anti-F4/80 and anti-TIE2 antibodies. Boxed regions are shown in magnified views in the right panel. Scale bars, 100 μm. (C-E) Double immunofluorescence staining of a representative metastatic LN section using anti-TIE2 and anti-F4/80 antibodies (C), anti-F4/80 and anti-CD31 antibodies (D), and anti-TIE2 and anti-CD31 antibodies (E). Scale bars (left to right), 50, 20, and 20 μm.
Fig. 4
Assessment of CD163, TIE2, and CD31 expression in reactive hyperplastic lymph nodes and metastatic lymph nodes from healthy and breast tumor-bearing human subjects. (A) Immunohistochemical images for anti-CD163, anti-TIE2, and anti-CD31 antibodies are shown for representative reactive hyperplastic lymph nodes from human subjects. Boxed regions are shown as magnified views in the right panel. Scale bars, 500 μm (left) and 50 μm (right). (B) Immunohisto-chemical images for anti-CD163, anti-TIE2, and anti-CD31 antibodies are shown for metastatic lymph nodes from breast tumor-bearing human subjects. Boxed regions are shown as magnified views in the right panel. Scale bars, 500 μm (left) and 50 μm (right). (C-E) Double immunofluorescence staining of two representative metastatic lymph node sections from a breast tumor-bearing human subject using anti-TIE2 and anti-CD163 antibodies (C), anti-CD31 and anti-CD163 antibodies (D), and anti-CD31 and anti-TIE2 antibodies (E), Scale bars (left to right), 20, 50, and 20 μm.
Similar articles
- Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters.
Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. Bolat F, et al. J Exp Clin Cancer Res. 2006 Sep;25(3):365-72. J Exp Clin Cancer Res. 2006. PMID: 17167977 - Splenectomy reduces lung metastases and tumoral and metastatic niche inflammation.
Stöth M, Freire Valls A, Chen M, Hidding S, Knipper K, Shen Y, Klose J, Ulrich A, Ruiz de Almodovar C, Schneider M, Schmidt T. Stöth M, et al. Int J Cancer. 2019 Nov 1;145(9):2509-2520. doi: 10.1002/ijc.32378. Epub 2019 May 13. Int J Cancer. 2019. PMID: 31034094 - The Selective Tie2 Inhibitor Rebastinib Blocks Recruitment and Function of Tie2Hi Macrophages in Breast Cancer and Pancreatic Neuroendocrine Tumors.
Harney AS, Karagiannis GS, Pignatelli J, Smith BD, Kadioglu E, Wise SC, Hood MM, Kaufman MD, Leary CB, Lu WP, Al-Ani G, Chen X, Entenberg D, Oktay MH, Wang Y, Chun L, De Palma M, Jones JG, Flynn DL, Condeelis JS. Harney AS, et al. Mol Cancer Ther. 2017 Nov;16(11):2486-2501. doi: 10.1158/1535-7163.MCT-17-0241. Epub 2017 Aug 24. Mol Cancer Ther. 2017. PMID: 28838996 Free PMC article. - The role of tumor-associated macrophages in breast cancer progression (review).
Obeid E, Nanda R, Fu YX, Olopade OI. Obeid E, et al. Int J Oncol. 2013 Jul;43(1):5-12. doi: 10.3892/ijo.2013.1938. Epub 2013 May 14. Int J Oncol. 2013. PMID: 23673510 Free PMC article. Review. - The role of tumor-associated macrophage in breast cancer biology.
Choi J, Gyamfi J, Jang H, Koo JS. Choi J, et al. Histol Histopathol. 2018 Feb;33(2):133-145. doi: 10.14670/HH-11-916. Epub 2017 Jul 6. Histol Histopathol. 2018. PMID: 28681373 Review.
Cited by
- Tumor-associated macrophages: an accomplice in solid tumor progression.
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Chen Y, et al. J Biomed Sci. 2019 Oct 20;26(1):78. doi: 10.1186/s12929-019-0568-z. J Biomed Sci. 2019. PMID: 31629410 Free PMC article. Review. - Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy.
Glabman RA, Choyke PL, Sato N. Glabman RA, et al. Cancers (Basel). 2022 Aug 12;14(16):3906. doi: 10.3390/cancers14163906. Cancers (Basel). 2022. PMID: 36010899 Free PMC article. Review. - NK Cell Anti-Tumor Surveillance in a Myeloid Cell-Shaped Environment.
Russo E, Laffranchi M, Tomaipitinca L, Del Prete A, Santoni A, Sozzani S, Bernardini G. Russo E, et al. Front Immunol. 2021 Dec 17;12:787116. doi: 10.3389/fimmu.2021.787116. eCollection 2021. Front Immunol. 2021. PMID: 34975880 Free PMC article. Review. - Multiple influence of immune cells in the bone metastatic cancer microenvironment on tumors.
Chen S, Lei J, Mou H, Zhang W, Jin L, Lu S, Yinwang E, Xue Y, Shao Z, Chen T, Wang F, Zhao S, Chai X, Wang Z, Zhang J, Zhang Z, Ye Z, Li B. Chen S, et al. Front Immunol. 2024 Feb 23;15:1335366. doi: 10.3389/fimmu.2024.1335366. eCollection 2024. Front Immunol. 2024. PMID: 38464516 Free PMC article. Review. - Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis.
Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL. Corliss BA, et al. Microcirculation. 2016 Feb;23(2):95-121. doi: 10.1111/micc.12259. Microcirculation. 2016. PMID: 26614117 Free PMC article. Review.
References
- Giatromanolaki A, Koukourakis MI, Kakolyris S, Kaklamanis L, Barbatis K, O’Byrne KJ, Theodosssiou D, Harris AL, Gatter KC. Focal expression of thymidine phosphorylase associates with CD31 positive lymphocytic aggregation and local neoangiogenesis in non-small cell lung cancer. Anticancer Res. 1998;18:71–76. - PubMed
- Groblewska M, Mroczko B, Wereszczynska-Siemiatkowska U, Mysliwiec P, Kedra B, Szmitkowski M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clin Chem Lab Med. 2007;45:30–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous