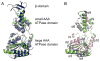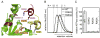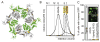The oligomeric state of the active Vps4 AAA ATPase - PubMed (original) (raw)
The oligomeric state of the active Vps4 AAA ATPase
Nicole Monroe et al. J Mol Biol. 2014.
Abstract
The cellular ESCRT (endosomal sorting complexes required for transport) pathway drives membrane constriction toward the cytosol and effects membrane fission during cytokinesis, endosomal sorting, and the release of many enveloped viruses, including the human immunodeficiency virus. A component of this pathway, the AAA ATPase Vps4, provides energy for pathway progression. Although it is established that Vps4 functions as an oligomer, subunit stoichiometry and other fundamental features of the functional enzyme are unclear. Here, we report that although some mutant Vps4 proteins form dodecameric assemblies, active wild-type Saccharomyces cerevisiae and Sulfolobus solfataricus Vps4 enzymes can form hexamers in the presence of ATP and ADP, as assayed by size-exclusion chromatography and equilibrium analytical ultracentrifugation. The Vta1p activator binds hexameric yeast Vps4p without changing the oligomeric state of Vps4p, implying that the active Vta1p-Vps4p complex also contains a single hexameric ring. Additionally, we report crystal structures of two different archaeal Vps4 homologs, whose structures and lattice interactions suggest a conserved mode of oligomerization. Disruption of the proposed hexamerization interface by mutagenesis abolished the ATPase activity of archaeal Vps4 proteins and blocked Vps4p function in S. cerevisiae. These data challenge the prevailing model that active Vps4 is a double-ring dodecamer, and argue that, like other type I AAA ATPases, Vps4 functions as a single ring with six subunits.
Keywords: AAA ATPase; AUC; CPS; EM; ESCRT; GFP; HIV budding; SSRL; Stanford Synchrotron Radiation Lightsource; analytical ultracentrifugation; carboxypeptidase S; electron microscopy; green fluorescent protein; multivesicular body; protein oligomerization.
© 2013. Published by Elsevier Ltd. All rights reserved.
Figures
Figure 1
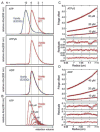
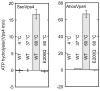
Oligomerization of S. cerevisiae Vps4p proteins. (A) Size exclusion chromatograms of wild-type Vps4p (red) and Vps4p(E233Q) (blue), injected at a concentration of 100 μM, in the presence of 2 mM magnesium chloride and 1 mM ATP, 0.2 mM ATPγS, or 1 mM ADP. Expected retention volumes for different oligomeric states of Vps4p based on molecular weight standards are indicated with dotted lines. Note that Vps4p(E233Q) elutes as a dodecamer in the presence of ATP, but as a hexamer in the presence of ADP, whereas wild-type Vps4p elutes as a hexamer in the presence of ATP, ATPγS or ADP. (B) Size exclusion chromatograms of wild-type Vps4p at concentrations ranging from 10 μM to 200 μM in the presence of 1 mM ATP. Note that the complex migrates more slowly at lower concentrations, indicating that Vps4p is in a rapid equilibrium between different oligomeric states. (C, D) Equilibrium analytical ultracentrifugation at 4 °C indicates that wild-type Vps4p is in a dimer-hexamer equilibrium in the presence of (C) 1 mM ATPγS or (D) 1 mM ADP. The interference signal from sedimentation data at 5000 rpm is plotted versus the distance from the axis of rotation (radius) as red symbols. Three different protein concentrations are displayed: 40 μM (upper), 20 μM (middle) and 10 μM (lower). The global fit to data obtained for three concentrations at rotor speeds of 3000 rpm (not shown) and 5000 rpm using a dimer-hexamer model is shown in black with residuals for all three concentrations displayed below.
Figure 2
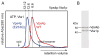
Wild type Vps4p remains hexameric and Vps4p(E233Q) remains dodecameric in the presence of Vta1p. (A) ATP-bound wild-type and E233Q Vps4p size exclusion chromatograms. Calculated retention volumes for 6:6, 6:12, and 12:12 Vps4p:Vta1p complexes are indicated. The void volume and the expected elution volume for the Vps4p hexamer are indicated by arrows. (B) SDS PAGE analysis of the peak fraction of wild-type Vps4p (48 kDa) in the presence of Vta1p (37 kDa) confirms that the peak represents a complex of both proteins.
Figure 3
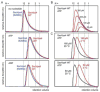
Characterization of SsoVps4 oligomerization by size exclusion chromatography. (A) Chromatograms of SsoVps4 (red) and SsoVps4(E206Q) (blue) injected at concentrations of 100 μM. Both proteins eluted as dodecamers in the absence of nucleotide but eluted as hexamers when the buffer was supplemented with 2 mM magnesium chloride and 1 mM ATP or ADP. (B) Chromatograms of different concentrations of SsoVps4. The shift in retention volume following injection at different protein concentrations indicates that SsoVps4 rapidly interconverts between multiple oligomeric states. (C) A shift of temperature from 4 °C (red) to 20 °C (black) reduces the elution volume: 160 μM (upper) and 80 μM (lower). Standard molecular weight markers displayed very similar retention volumes at the two temperatures.
Figure 4
Characterization of SsoVps4 oligomerization by equilibrium analytical ultracentrifugation. (A) SsoVps4(E206Q) in the absence of nucleotide. Data are shown from a 3500 rpm spin at three protein concentrations: 10 μM (upper), 5 μM (middle) and 2.5 μM (lower). The black line corresponds to an ideal species fit for dodecameric SsoVps4. Residuals are displayed below. (B) Wild-type SsoVps4 in the presence of 1 mM ATP at 5000 rpm. Three different protein concentrations are displayed: 47 μM (upper), 24 μM (middle) and 12 μM (lower). Interference scans at 3000 rpm for all concentrations were also included in the fit, but are not shown here. The fit obtained using a dimer-hexamer model is shown in black.
Figure 5
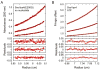
ATPase activity of SsoVps4 and AhosVps4 at 4 °C, 37 °C and 60 °C. Both proteins are active at 60 °C. Mutation of the Walker B glutamate in SsoVps4(E206Q) or AhosVps4(E200Q) abolishes ATPase activity.
Figure 6
Crystal structures of the ATPase cassettes of SsoVps4 and AhosVps4. (A) Superposition of SsoVps4 (green) and Vps4p (PDB ID: 3EIE , blue) yields an RMSD of 1.622 Å over 237 pairs of Cα atoms. (B) The structures of SsoVps4 (green) and AhosVps4 (pink) overlay with an RMSD of 0.492 Å on 206 pairs of Cα atoms.
Figure 7
The crystallographic interface between SsoVps4 molecules related by a 64 screw axis provides a model for the hexamer interface in solution. (A) Six symmetry-related molecules shown in separate colors viewed along (lower) and perpendicular to (upper) the 64 screw axis. (B) Detailed view of the contact boxed in panel A. Contacting residues are labeled and shown as sticks. Y121 and F328 form a π-stacking interaction at the center of the interface. (C) Size exclusion chromatograms of wild-type SsoVps4, SsoVps4(Y121D) and SsoVps4(F328A) in the presence of 1 mM ATP. (D) SsoVps4 ATPase activity assays performed at 60°C.
Figure 8
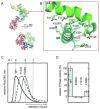
Arginine finger residues contribute to Vps4 oligomerization and are important for ATPase activity. (A) The nucleotide binding site of SsoVps4 (green) contains the conserved Walker A (brown), Walker B (orange) motifs. The neighboring subunit in the crystal is shown in yellow, and its position in the p97-like hexamer model is shown in magenta. Glutamate 206 and the potential arginine finger residues 262 and 263 are shown in stick representation. (B) Point mutations R262A and R263A destabilize the oligomer and (C) abolish ATPase activity.
Figure 9
Mutational analysis of the hexamer interface in yeast Vps4p. (A) L151 (orange spheres) and R352 (yellow spheres) map to the hexamer interface in the p97-like ring. (B) Vps4p(R352A) and Vps4p(L151D) do not form higher-order oligomers as shown by size exclusion chromatography in the presence of 1 mM ATP. (C) Vacuolar protein sorting of the model cargo CPS-GFP into the vacuole lumen is severely impaired in yeast cells expressing interface mutants Vps4p(L151D) or Vps4p(R352A) as compared to cells containing wild-type Vps4p. Insets show control examples of successful (upper) and unsuccessful (lower) CPS-GFP sorting.
Similar articles
- Biochemical and structural studies of yeast Vps4 oligomerization.
Gonciarz MD, Whitby FG, Eckert DM, Kieffer C, Heroux A, Sundquist WI, Hill CP. Gonciarz MD, et al. J Mol Biol. 2008 Dec 26;384(4):878-95. doi: 10.1016/j.jmb.2008.09.066. Epub 2008 Oct 4. J Mol Biol. 2008. PMID: 18929572 Free PMC article. - Binding of Substrates to the Central Pore of the Vps4 ATPase Is Autoinhibited by the Microtubule Interacting and Trafficking (MIT) Domain and Activated by MIT Interacting Motifs (MIMs).
Han H, Monroe N, Votteler J, Shakya B, Sundquist WI, Hill CP. Han H, et al. J Biol Chem. 2015 May 22;290(21):13490-9. doi: 10.1074/jbc.M115.642355. Epub 2015 Apr 1. J Biol Chem. 2015. PMID: 25833946 Free PMC article. - Structural role of the Vps4-Vta1 interface in ESCRT-III recycling.
Yang D, Hurley JH. Yang D, et al. Structure. 2010 Aug 11;18(8):976-84. doi: 10.1016/j.str.2010.04.014. Structure. 2010. PMID: 20696398 Free PMC article. - Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes.
McCullough J, Frost A, Sundquist WI. McCullough J, et al. Annu Rev Cell Dev Biol. 2018 Oct 6;34:85-109. doi: 10.1146/annurev-cellbio-100616-060600. Epub 2018 Aug 10. Annu Rev Cell Dev Biol. 2018. PMID: 30095293 Free PMC article. Review. - The role of VPS4 in ESCRT-III polymer remodeling.
Caillat C, Maity S, Miguet N, Roos WH, Weissenhorn W. Caillat C, et al. Biochem Soc Trans. 2019 Feb 28;47(1):441-448. doi: 10.1042/BST20180026. Epub 2019 Feb 19. Biochem Soc Trans. 2019. PMID: 30783012 Review.
Cited by
- Vps4 stimulatory element of the cofactor Vta1 contacts the ATPase Vps4 α7 and α9 to stimulate ATP hydrolysis.
Davies BA, Norgan AP, Payne JA, Schulz ME, Nichols MD, Tan JA, Xu Z, Katzmann DJ. Davies BA, et al. J Biol Chem. 2014 Oct 10;289(41):28707-18. doi: 10.1074/jbc.M114.580696. Epub 2014 Aug 27. J Biol Chem. 2014. PMID: 25164817 Free PMC article. - Mechanism of Vps4 hexamer function revealed by cryo-EM.
Su M, Guo EZ, Ding X, Li Y, Tarrasch JT, Brooks CL 3rd, Xu Z, Skiniotis G. Su M, et al. Sci Adv. 2017 Apr 14;3(4):e1700325. doi: 10.1126/sciadv.1700325. eCollection 2017 Apr. Sci Adv. 2017. PMID: 28439563 Free PMC article. - Asymmetric ring structure of Vps4 required for ESCRT-III disassembly.
Caillat C, Macheboeuf P, Wu Y, McCarthy AA, Boeri-Erba E, Effantin G, Göttlinger HG, Weissenhorn W, Renesto P. Caillat C, et al. Nat Commun. 2015 Dec 3;6:8781. doi: 10.1038/ncomms9781. Nat Commun. 2015. PMID: 26632262 Free PMC article. - Vps4 substrate binding and coupled mechanisms of Vps4p substrate recruitment and release from autoinhibition.
Wienkers HJ, Han H, Whitby FG, Hill CP. Wienkers HJ, et al. bioRxiv [Preprint]. 2024 Sep 7:2024.09.07.611824. doi: 10.1101/2024.09.07.611824. bioRxiv. 2024. PMID: 39282404 Free PMC article. Preprint. - Structure of Vps4 with circular peptides and implications for translocation of two polypeptide chains by AAA+ ATPases.
Han H, Fulcher JM, Dandey VP, Iwasa JH, Sundquist WI, Kay MS, Shen PS, Hill CP. Han H, et al. Elife. 2019 Jun 11;8:e44071. doi: 10.7554/eLife.44071. Elife. 2019. PMID: 31184588 Free PMC article.
References
- Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–12. - PubMed
- Caballe A, Martin-Serrano J. ESCRT machinery and cytokinesis: the road to daughter cell separation. Traffic. 2011;12:1318–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 GM082545/GM/NIGMS NIH HHS/United States
- R01 GM112080/GM/NIGMS NIH HHS/United States
- R01 AI051174/AI/NIAID NIH HHS/United States
- P30CA042014/CA/NCI NIH HHS/United States
- T32 AI055434/AI/NIAID NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases