Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.2337/dc13-1545. Epub 2013 Oct 29.
Collaborators, Affiliations
- PMID: 24170759
- PMCID: PMC3931381
- DOI: 10.2337/dc13-1545
Randomized Controlled Trial
Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial
Michael E Miller et al. Diabetes Care. 2014.
Abstract
Objective: We explore the effect of randomized treatment, comparing intensive to standard glucose-lowering strategies on major cardiovascular outcomes, death, and severe adverse events in older versus younger participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.
Research design and methods: Participants with type 2 diabetes (n = 10,251) with a mean age of 62 years, a median duration of diabetes of 10 years, and a median A1C of 8.1% (65 mmol/mol) were randomized to treatment strategies targeting either A1C <6.0% (42 mmol/mol) or 7.0-7.9% (53-63 mmol/mol) and followed for a mean of 3.7 years. Outcomes were analyzed within subgroups defined by baseline age (<65 vs. ≥65 years).
Results: Older and younger ACCORD participants achieved similar intensive-arm A1C levels and between-arm A1C differences. Within the older subgroup, similar hazards of the cardiovascular primary outcome and total mortality were observed in the two arms. While there was no intervention effect on cardiovascular mortality in the older subgroup, there was an increased risk in the intensive arm for the younger subgroup (older hazard ratio [HR] = 0.97; younger HR = 1.71; P = 0.03). Regardless of intervention arm, the older subgroup experienced higher annualized rates of severe hypoglycemia (4.45% intensive and 1.36% standard) than the younger subgroup (2.45% intensive and 0.80% standard).
Conclusions: Intensive glucose lowering increased the risk of cardiovascular disease and total mortality in younger participants, whereas it had a neutral effect in older participants. The intensive to standard relative risk of severe hypoglycemia was similar in both age subgroups, with higher absolute rates in older participants within both treatment arms.
Trial registration: ClinicalTrials.gov NCT00000620.
Figures
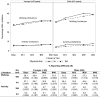
Figure 1
Horizontal bars represent the 95% CI on the HR. Hosp, hospitalization.
Figure 2
Plots represent the proportion of participants reporting difficulty with either walking or activities at each follow-up visit. In the table below the figure, the number of participants providing data at each time point is presented in parentheses, after the percentage of participants reporting difficulty. M12, month 12; M24, month 24; M36, month 36; M48, month 48; Int, intensive; Std, standard; Gly, glycemia.
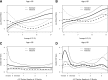
Figure 3
Spline curves of the risk of all-cause mortality with the two treatment strategies. A and B: The linear part of the PH model for average A1C from 6.0 to 9.0% (42 to 75 mmol/mol) for participants aged <65 and ≥65 years at randomization, respectively. For clarity, the figure omits values <6 (42 mmol/mol) and >9% (75 mmol/mol); approximately 5% of deaths are excluded from the plot at the lower and also at the higher end of the A1C range, but these data are included in the models. The plotted values are relative to a standard participant, aged <65 years at randomization and at an A1C of 6%. The dashed lines represent estimates and CIs for standard participants, solid lines are for intensive participants. C and D: The results from a Poisson regression model of all-cause mortality rates by treatment and age subgroup for the whole period of follow-up, over a range of decreases in A1C from baseline in the first year of treatment (as %A1C). The figures omit values beyond the 5th and 95th percentiles of A1C changes. The full range of values was from 6.8% (51 mmol/mol; an increase) to 7.4% (57 mmol/mol; a decrease) from baseline. The calculations used a Poisson regression model with data from model 3 of Riddle et al. (20).
Comment in
- Intensive glucose-lowering results in increased cardiovascular mortality in younger but not older individuals with type 2 diabetes.
Giorgino F. Giorgino F. Evid Based Med. 2014 Dec;19(6):210. doi: 10.1136/ebmed-2014-110010. Epub 2014 Jul 15. Evid Based Med. 2014. PMID: 25028606 No abstract available.
Similar articles
- The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study.
Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. Bonds DE, et al. BMJ. 2010 Jan 8;340:b4909. doi: 10.1136/bmj.b4909. BMJ. 2010. PMID: 20061358 Free PMC article. Clinical Trial. - The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial.
Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V. Hempe JM, et al. Diabetes Care. 2015 Jun;38(6):1067-74. doi: 10.2337/dc14-1844. Epub 2015 Apr 17. Diabetes Care. 2015. PMID: 25887355 Free PMC article. Clinical Trial. - The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study.
Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, Feinglos MN, Ismail-Beigi F, Largay JF, O'Connor PJ, Paul T, Savage PJ, Schubart UK, Sood A, Genuth S; ACCORD Investigators. Miller ME, et al. BMJ. 2010 Jan 8;340:b5444. doi: 10.1136/bmj.b5444. BMJ. 2010. PMID: 20061360 Free PMC article. Clinical Trial. - Effect of Intensive Versus Standard Blood Glucose Control in Patients With Type 2 Diabetes Mellitus in Different Regions of the World: Systematic Review and Meta-analysis of Randomized Controlled Trials.
Sardar P, Udell JA, Chatterjee S, Bansilal S, Mukherjee D, Farkouh ME. Sardar P, et al. J Am Heart Assoc. 2015 May 5;4(5):e001577. doi: 10.1161/JAHA.114.001577. J Am Heart Assoc. 2015. PMID: 25944874 Free PMC article. Review. - Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials.
Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Boussageon R, et al. BMJ. 2011 Jul 26;343:d4169. doi: 10.1136/bmj.d4169. BMJ. 2011. PMID: 21791495 Free PMC article. Review.
Cited by
- Frailty Detection in Older Adults with Diabetes: A Scoping Review of Assessment Tools and Their Link to Key Clinical Outcomes.
Guevara E, Simó-Servat A, Perea V, Quirós C, Puig-Jové C, Formiga F, Barahona MJ. Guevara E, et al. J Clin Med. 2024 Sep 9;13(17):5325. doi: 10.3390/jcm13175325. J Clin Med. 2024. PMID: 39274537 Free PMC article. Review. - Potential Benefits of Continuous Glucose Monitoring for Predicting Vascular Outcomes in Type 2 Diabetes: A Rapid Review of Primary Research.
Jadav RK, Yee KC, Turner M, Mortazavi R. Jadav RK, et al. Healthcare (Basel). 2024 Aug 4;12(15):1542. doi: 10.3390/healthcare12151542. Healthcare (Basel). 2024. PMID: 39120245 Free PMC article. Review. - A deprescribing programme aimed to optimise blood glucose-lowering medication in older people with type 2 diabetes mellitus, the OMED2-study: the study protocol for a randomised controlled trial.
Andriessen C, Blom MT, van Hoek BACE, de Boer AW, Denig P, de Wit GA, Swart K, de Rooij-Peek A, van Marum RJ, Hugtenburg JG, Slottje P, van Raalte D, van Bloemendaal L, Herings R, Nijpels G, Vos RC, Elders PJM. Andriessen C, et al. Trials. 2024 Jul 25;25(1):505. doi: 10.1186/s13063-024-08249-9. Trials. 2024. PMID: 39049109 Free PMC article. - Glycemic control and dementia risk in patients aged above and below 75 years.
Jung HH. Jung HH. Diabetol Int. 2024 Jan 8;15(2):244-252. doi: 10.1007/s13340-023-00684-4. eCollection 2024 Apr. Diabetol Int. 2024. PMID: 38524931 - Glycemic control and cardiovascular outcomes in patients with diabetes and coronary artery disease according to triglyceride-glucose index: a large-scale cohort study.
Lin Z, He J, Yuan S, Song C, Bian X, Yang M, Dou K. Lin Z, et al. Cardiovasc Diabetol. 2024 Jan 6;23(1):11. doi: 10.1186/s12933-023-02112-y. Cardiovasc Diabetol. 2024. PMID: 38184572 Free PMC article.
References
- Department of Health and Human Services CfDCaP National diabetes fact sheet; national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, Centers for Disease Control and Prevention, 2011
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 - PubMed
- Panzram G. Mortality and survival in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1987;30:123–131 - PubMed
- Stengård JH, Tuomilehto J, Pekkanen J, et al. Diabetes mellitus, impaired glucose tolerance and mortality among elderly men: the Finnish cohorts of the Seven Countries Study. Diabetologia 1992;35:760–765 - PubMed
- Katakura M, Naka M, Kondo T, et al. Prospective analysis of mortality, morbidity, and risk factors in elderly diabetic subjects: Nagano study. Diabetes Care 2003;26:638–644 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01HC95183/HC/NHLBI NIH HHS/United States
- Y01 HC001010/HC/NHLBI NIH HHS/United States
- N01HC95178/HC/NHLBI NIH HHS/United States
- N01-HC-95180/HC/NHLBI NIH HHS/United States
- Y01 HC009035/HC/NHLBI NIH HHS/United States
- N01-HC-95184/HC/NHLBI NIH HHS/United States
- P30-AG21332/AG/NIA NIH HHS/United States
- N01-HC-95183/HC/NHLBI NIH HHS/United States
- N01HC95182/HC/NHLBI NIH HHS/United States
- N01-HC-95179/HC/NHLBI NIH HHS/United States
- N01 HC-95178/HC/NHLBI NIH HHS/United States
- N01-HC-95181/HC/NHLBI NIH HHS/United States
- N01HC95179/HC/NHLBI NIH HHS/United States
- P30 AG021332/AG/NIA NIH HHS/United States
- N01HC95180/HC/NHLBI NIH HHS/United States
- N01HC95181/HC/NHLBI NIH HHS/United States
- N01HC95184/HC/NHLBI NIH HHS/United States
- N01-HC-95182/HC/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical