Platelets and Alzheimer's disease: Potential of APP as a biomarker - PubMed (original) (raw)
Review
Platelets and Alzheimer's disease: Potential of APP as a biomarker
Geneviève Evin et al. World J Psychiatry. 2012.
Abstract
Platelets are the first peripheral source of amyloid precursor protein (APP). They possess the proteolytic machinery to produce Aβ and fragments similar to those produced in neurons, and thus offer an ex-vivo model to study APP processing and changes associated with Alzheimer's disease (AD). Platelet process APP mostly through the α-secretase pathway to release soluble APP (sAPP). They produce small amounts of Aβ, predominantly Aβ40 over Aβ42. sAPP and Aβ are stored in α-granules and are released upon platelet activation by thrombin and collagen, and agents inducing platelet degranulation. A small proportion of full-length APP is present at the platelet surface and this increases by 3-fold upon platelet activation. Immunoblotting of platelet lysates detects APP as isoforms of 130 kDa and 106-110 kDa. The ratio of these of APP isoforms is significantly lower in patients with AD and mild cognitive impairment (MCI) than in healthy controls. This ratio follows a decrease that parallels cognitive decline and can predict conversion from MCI to AD. Alterations in the levels of α-secretase ADAM10 and in the enzymatic activities of α- and β-secretase observed in platelets of patients with AD are consistent with increased processing through the amyloidogenic pathway. β-APP cleaving enzyme activity is increased by 24% in platelet membranes of patients with MCI and by 17% in those with AD. Reports of changes in platelet APP expression with MCI and AD have been promising so far and merit further investigation as the search for blood biomarkers in AD, in particular at the prodromal stage, remains a priority and a challenge.
Keywords: Alzheimer’s disease; Amyloid precursor protein; Aβ amyloid; Biomarker; Platelet; Protease-nexin 2; Secretase; β-amyloid precursor protein cleaving enzyme.
Figures
Figure 1
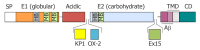
Schematic diagram of the amyloid precursor protein. The diagram represents the structural domain arrangement of the amyloid precursor protein (APP) gene products. All domains are expressed in the APP770 isoform. The OX-2 domain is missing in APP751, whereas both the Kunitz protease inhibitor domain (KPI) and the OX-2 domain are missing in APP695. SP: Signal peptide; E1: Ectodomain 1; E2: Ectodomain 2; TMD: Transmembrane domain; CD: Cytosolic domain; OX-2: Domain with homology to OX-2 leukocyte antigen; Ex 15: Exon 15 product; HB: Heparin-binding domain; MB: Metal ion-binding domain.
Figure 2
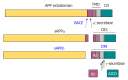
Proteolytic processing of amyloid precursor protein by the secretases. In a non-amyloidogenic pathway, α-secretase cleaves amyloid precursor protein (APP) within the Aβ domain to release the α-cleaved soluble N-terminal fragment, sAPPα. β-Amyloid precursor cleaving enzyme (BACE) cleaves APP at the N-terminus of Aβ to release the β-cleaved soluble N-terminal fragment sAPPβ, and thereby initiates the amyloidogenic pathway. The remaining 99 amino acid-long C-terminal fragment (C99) is further processed at multiple sites by γ-secretase to release the APP intracellular domain (AICD) in the cytosol and Aβ peptides in the extracellular space.
Similar articles
- Platelet-derived secreted amyloid-precursor protein-β as a marker for diagnosing Alzheimer's disease.
Marksteiner J, Humpel C. Marksteiner J, et al. Curr Neurovasc Res. 2013 Nov;10(4):297-303. doi: 10.2174/15672026113109990022. Curr Neurovasc Res. 2013. PMID: 23937201 Free PMC article. - Platelet amyloid precursor protein processing: a bio-marker for Alzheimer's disease.
Tang K, Hynan LS, Baskin F, Rosenberg RN. Tang K, et al. J Neurol Sci. 2006 Jan 15;240(1-2):53-8. doi: 10.1016/j.jns.2005.09.002. Epub 2005 Oct 26. J Neurol Sci. 2006. PMID: 16256140 Free PMC article. - Processing of the platelet amyloid precursor protein in the mild cognitive impairment (MCI).
Bermejo-Bescós P, Martín-Aragón S, Jiménez-Aliaga K, Benedí J, Felici E, Gil P, Ribera JM, Villar AM. Bermejo-Bescós P, et al. Neurochem Res. 2013 Jul;38(7):1415-23. doi: 10.1007/s11064-013-1039-7. Epub 2013 Apr 11. Neurochem Res. 2013. PMID: 23575575 - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) as a biological candidate marker of Alzheimer's disease.
Hampel H, Shen Y. Hampel H, et al. Scand J Clin Lab Invest. 2009;69(1):8-12. doi: 10.1080/00365510701864610. Scand J Clin Lab Invest. 2009. PMID: 18609117 Review.
Cited by
- One immune cell to bind them all: platelet contribution to neurodegenerative disease.
Rodriguez Moore G, Melo-Escobar I, Stegner D, Bracko O. Rodriguez Moore G, et al. Mol Neurodegener. 2024 Sep 27;19(1):65. doi: 10.1186/s13024-024-00754-4. Mol Neurodegener. 2024. PMID: 39334369 Free PMC article. Review. - Beta-Amyloid Enhances Vessel Formation in Organotypic Brain Slices Connected to Microcontact Prints.
Steiner K, Humpel C. Steiner K, et al. Biomolecules. 2023 Dec 19;14(1):3. doi: 10.3390/biom14010003. Biomolecules. 2023. PMID: 38275744 Free PMC article. - A Mild Dose of Aspirin Promotes Hippocampal Neurogenesis and Working Memory in Experimental Ageing Mice.
Vergil Andrews JF, Selvaraj DB, Kumar A, Roshan SA, Anusuyadevi M, Kandasamy M. Vergil Andrews JF, et al. Brain Sci. 2023 Jul 21;13(7):1108. doi: 10.3390/brainsci13071108. Brain Sci. 2023. PMID: 37509038 Free PMC article. - A Novel Longitudinal Phenotype-Genotype Association Study Based on Deep Feature Extraction and Hypergraph Models for Alzheimer's Disease.
Kong W, Xu Y, Wang S, Wei K, Wen G, Yu Y, Zhu Y. Kong W, et al. Biomolecules. 2023 Apr 23;13(5):728. doi: 10.3390/biom13050728. Biomolecules. 2023. PMID: 37238598 Free PMC article. - Modulating heart rate oscillation affects plasma amyloid beta and tau levels in younger and older adults.
Min J, Rouanet J, Martini AC, Nashiro K, Yoo HJ, Porat S, Cho C, Wan J, Cole SW, Head E, Nation DA, Thayer JF, Mather M. Min J, et al. Sci Rep. 2023 Mar 9;13(1):3967. doi: 10.1038/s41598-023-30167-0. Sci Rep. 2023. PMID: 36894565 Free PMC article.
References
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. - PubMed
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
- Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 1993;16:460–465. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources