Neurotransmitter release: the last millisecond in the life of a synaptic vesicle - PubMed (original) (raw)
Review
Neurotransmitter release: the last millisecond in the life of a synaptic vesicle
Thomas C Südhof. Neuron. 2013.
Abstract
During an action potential, Ca(2+) entering a presynaptic terminal triggers synaptic vesicle exocytosis and neurotransmitter release in less than a millisecond. How does Ca(2+) stimulate release so rapidly and precisely? Work over the last decades revealed that Ca(2+) binding to synaptotagmin triggers release by stimulating synaptotagmin binding to a core fusion machinery composed of SNARE and SM proteins that mediates membrane fusion during exocytosis. Complexin adaptor proteins assist synaptotagmin by activating and clamping this core fusion machinery. Synaptic vesicles containing synaptotagmin are positioned at the active zone, the site of vesicle fusion, by a protein complex containing RIM proteins. RIM proteins activate docking and priming of synaptic vesicles and simultaneously recruit Ca(2+) channels to active zones, thereby connecting in a single complex primed synaptic vesicles to Ca(2+) channels. This architecture allows direct flow of Ca(2+) ions from Ca(2+) channels to synaptotagmin, which then triggers fusion, thus mediating tight millisecond coupling of an action potential to neurotransmitter release.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
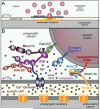
Figure 1. Organization of the presynaptic release machinery
A. Drawing of a synapse with synaptic vesicles (SV), an active zone containing Ca2+-channels (blue), and a postsynaptic cluster of receptors (orange). One vesicle in the active zone is depicted in the process of fusing, with red neurotransmitters emitting from the fusion pore. B. Schematic of the molecular machinery mediating Ca2+-triggered vesicle fusion. The drawing depicts a segment of a docked synaptic vesicle on the top right, and the presynaptic active zone in the middle. The three functional elements of the neurotransmitter release machinery are depicted from right to left. On the right, the core fusion machine composed of the SNARE/SM protein complex is shown; this machine comprises the SNARE proteins synaptobrevin/VAMP, syntaxin-1, and SNAP-25 and the SM protein Munc18-1. The Ca2+-sensor synaptotagmin-1 is depicted in the middle; it is composed of a short intravesicular sequence, a single transmembrane region, and two cytoplasmic C2-domains that bind Ca2+, and it functions using complexin (bound to the SNARE complex) as an assistant. The active zone protein complex containing RIM, Munc13, and RIM-BP, and a Ca2+-channel in the presynaptic plasma membrane is shown on the left. In this protein complex, RIM binding to specific target proteins coordinates all three functions of the active zone: RIM binding to vesicular rab proteins (Rab3 and Rab27 isoforms) mediates vesicle docking; RIM binding to the central priming factor Munc13 activates vesicle priming; and RIM binding to the Ca2+-channel, both directly and indirectly via RIM-BP, recruits the Ca2+-channels within 100 nm of the docked vesicles for fast excitation-secretion coupling. The overall design of the neurotransmitter release machinery depicted here enables in a single nanodevice fast and efficient triggering of release in response to an action potential by combining a fusion machine with a Ca2+-trigger and an active zone protein complex that positions all elements into appropriate proximity (modified from Kaeser et al., 2011).
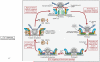
Figure 2. Schematic of the SNARE/SM protein cycle mediating fusion, and the role of synaptotagmin and complexin in Ca2+-triggering of fusion
SNARE and SM proteins undergo a cycle of assembly and disassembly, such that the vesicular SNARE protein synaptobrevin assembles during priming into a trans-SNARE complex with the plasma membrane SNARE proteins syntaxin-1 and SNAP-25. Prior to SNARE-complex assembly, syntaxin-1 is present in a closed conformation in which its Habc-domain folds back onto its SNARE motif; this conformation precludes SNARE-complex assembly, and syntaxin-1 has to ‘open’ for SNARE-complex assembly to initiate. Prior to SNARE-complex assembly, Munc18-1 is associated with monomeric syntaxin-1 when syntaxin-1 is in a closed conformation; as syntaxin-1 opens during SNARE-complex assembly, Munc18-1 alters the mode of its binding to syntaxin-1 by binding to assembling trans-SNARE complexes via interacting with the syntaxin-1 N-peptide. Once SNARE complexes are partially assembled, complexin binds to further increase their priming. The ‘superprimed’ SNARE/SM protein complexes are then substrate for Ca2+-triggered fusion pore opening by Ca2+-binding to synaptotagmin, which causes an interaction of synaptotagmin with SNAREs and phospholipids. However, even before Ca2+-triggering synaptotagmin likely at least partly interacts with the fusion machinery as evidenced by the unclamping of spontaneous mini release in Syt1 knockout neurons. After fusion-pore opening, the resulting cis-SNARE complexes are disassembled by the NSF/SNAP ATPases, and vesicles are recycled, refilled with neurotransmitters, and reused for release (modified from Südhof, 2013).
Figure 3. Energy landscape of fusion and proposed role of the SM protein Munc18-1 in promoting fusion-pore opening
A. Schematic diagram of the energy level of a vesicle that is docked, primed, and fused. The diagram illustrates that partial SNARE-complex assembly during priming is proposed to provide most of the energy required for fusion, such that Ca2+-triggering only adds a small amount of additional energy to induce fusion-pore opening. B. Model of Munc18-1 function in fusion. Prior to priming of docked vesicles, Munc18-1 is bound to the closed conformation of syntaxin-1; this interaction is primarily regulatory to maintain a defined rate of entry into the fusion reaction, and additionally serves for the mutual stabilization of Munc18-1 and syntaxin-1 for each other (Gerber et al., 2008; Zhou et al., 2013a). Partial SNARE-complex assembly during priming (middle) is associated with a dramatic conformational change in syntaxin-1 which has to open, and in Munc18-1 whose binding changes from that to a closed syntaxin-1 conformation to binding to the open syntaxin-1 conformation via the N-peptide of syntaxin-1 (Dulubova et al., 2007). Full SNARE-complex assembly (right) produces fusion-pore opening. Munc18-1 is proposed to ride on the assembly SNARE complexes throughout the fusion reaction, and to couple the approximation of the membranes produced by the energy released by SNARE-complex assembly to fusion, possibly by mediating phospholipid mixing.
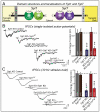
Figure 4. Complementary roles of synaptotagmin-1 (Syt1) and -7 (Syt7) Ca2+-sensors in triggering synchronous and asynchronous neurotransmitter release
A. Domain structures and localizations of Syt1 as the paradigmatic fast synaptotagmin isoform, and of Syt7 as the slow synaptotagmin isoform. Syt1 and Syt7 have identical domain structures, except that Syt1 contains an N-terminal N-glycosylation site in the vesicle that is lacking from Syt7, and that Syt1 is primarily localized to synaptic vesicles where Syt7 is primarily absent from synaptic vesicles. B. Inhibitory postsynaptic currents (IPSCs) measured in response to isolated action potentials in cultured hippocampal neurons from Syt1 knockout mice that were either infected with a control lentivirus, a lentivirus overexpressing wild-type Syt7, a lentivirus expressing a Syt7 shRNA that blocks most Syt7 expression, a lentivirus co-expressing the Syt7 shRNA together with wild-type Syt7, or a lentivirus co-expressing the Syt7 shRNA with a mutant Syt7 in which the C2A- and C2B-domain Ca2+-binding sites were mutated. Representative traces are shown on the left, and summary graphs on the right. Data shown are means ± SEM; numbers in the bars indicate the numbers of neurons/cultures analyzed. Statistical significance was assessed by one-way ANOVA comparing all test conditions to the control (**, p<0.01; ***, p<0.001). C. Same as B, except that IPSCs were measured in response to a 10 Hz 1 sec stimulus train. Data and figure were adapted from Bacaj et al. (2013).
Figure 5. Schematic representation of different synaptic Ca2+-controlled neurotransmitter release pathways
After docking and priming of synaptic vesicles mediated by the active zone protein complex containing RIM proteins as central elements, vesicles are further ‘superprimed’ by binding of complexin to the assembling SNARE complexes. The superprimed vesicles are the substrates of fast (Syt1/Syt2/Syt7) and slow (Syt7) synaptotagmins as synaptic Ca2+-sensors. Fast and slow synaptotagmins are differentially expressed, compete with each other, and are further regulated by alternative splicing and phosphorylation, such that synapses differ in the amount and short-term plasticity of Ca2+-induced release. In the absence of complexin or the fast synaptotagmin Ca2+-sensors, an alternative nonphysiological pathway becomes enabled (dotted line) whereby an as yet unidentified Ca2+-sensor with a Ca2+-cooperativity that differs from that of synaptotagmins mediates spontaneous ‘mini’ release. Key proteins are listed in the drawing at their points of action; SNARE and SM proteins are not shown for clarity sake, but are obviously vital components for the entire fusion reaction.
Similar articles
- The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis.
Zhou Q, Zhou P, Wang AL, Wu D, Zhao M, Südhof TC, Brunger AT. Zhou Q, et al. Nature. 2017 Aug 24;548(7668):420-425. doi: 10.1038/nature23484. Epub 2017 Aug 16. Nature. 2017. PMID: 28813412 Free PMC article. - A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis.
Tang J, Maximov A, Shin OH, Dai H, Rizo J, Südhof TC. Tang J, et al. Cell. 2006 Sep 22;126(6):1175-87. doi: 10.1016/j.cell.2006.08.030. Cell. 2006. PMID: 16990140 - SNAP25 disease mutations change the energy landscape for synaptic exocytosis due to aberrant SNARE interactions.
Kádková A, Murach J, Østergaard M, Malsam A, Malsam J, Lolicato F, Nickel W, Söllner TH, Sørensen JB. Kádková A, et al. Elife. 2024 Feb 27;12:RP88619. doi: 10.7554/eLife.88619. Elife. 2024. PMID: 38411501 Free PMC article. - Physical link and functional coupling of presynaptic calcium channels and the synaptic vesicle docking/fusion machinery.
Sheng ZH, Westenbroek RE, Catterall WA. Sheng ZH, et al. J Bioenerg Biomembr. 1998 Aug;30(4):335-45. doi: 10.1023/a:1021985521748. J Bioenerg Biomembr. 1998. PMID: 9758330 Review. - The Core Complex of the Ca2+-Triggered Presynaptic Fusion Machinery.
Brunger AT, Leitz J. Brunger AT, et al. J Mol Biol. 2023 Jan 15;435(1):167853. doi: 10.1016/j.jmb.2022.167853. Epub 2022 Oct 13. J Mol Biol. 2023. PMID: 36243149 Free PMC article. Review.
Cited by
- Profiles of secreted neuropeptides and catecholamines illustrate similarities and differences in response to stimulation by distinct secretagogues.
Podvin S, Bundey R, Toneff T, Ziegler M, Hook V. Podvin S, et al. Mol Cell Neurosci. 2015 Sep;68:177-85. doi: 10.1016/j.mcn.2015.06.008. Epub 2015 Jun 16. Mol Cell Neurosci. 2015. PMID: 26092702 Free PMC article. - A unique C2 domain at the C terminus of Munc13 promotes synaptic vesicle priming.
Padmanarayana M, Liu H, Michelassi F, Li L, Betensky D, Dominguez MJ, Sutton RB, Hu Z, Dittman JS. Padmanarayana M, et al. Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2016276118. doi: 10.1073/pnas.2016276118. Proc Natl Acad Sci U S A. 2021. PMID: 33836576 Free PMC article. - Spontaneous vesicle recycling in the synaptic bouton.
Truckenbrodt S, Rizzoli SO. Truckenbrodt S, et al. Front Cell Neurosci. 2014 Dec 8;8:409. doi: 10.3389/fncel.2014.00409. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25538561 Free PMC article. Review. - Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs.
Hoerndli FJ, Wang R, Mellem JE, Kallarackal A, Brockie PJ, Thacker C, Madsen DM, Maricq AV. Hoerndli FJ, et al. Neuron. 2015 Apr 22;86(2):457-74. doi: 10.1016/j.neuron.2015.03.011. Epub 2015 Apr 2. Neuron. 2015. PMID: 25843407 Free PMC article. - Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: a narrative review.
Xu ZQ, Zhang WJ, Su DF, Zhang GQ, Miao CY. Xu ZQ, et al. Ann Transl Med. 2021 Mar;9(6):509. doi: 10.21037/atm-21-273. Ann Transl Med. 2021. PMID: 33850906 Free PMC article. Review.
References
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc-13 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. - PubMed
- Brose N, Petrenko AG, Südhof TC, Jahn R. Synaptotagmin: A Ca2+ sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH086403/MH/NIMH NIH HHS/United States
- R01 DA030379/DA/NIDA NIH HHS/United States
- R01 NS077906/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous