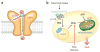A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis - PubMed (original) (raw)
Review
A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis
Shingo Kajimura et al. Annu Rev Physiol. 2014.
Abstract
Brown adipose tissue (BAT) is specialized to dissipate chemical energy in the form of heat as a defense against cold and excessive feeding. Interest in the field of BAT biology has exploded in the past few years because of the therapeutic potential of BAT to counteract obesity and obesity-related diseases, including insulin resistance. Much progress has been made, particularly in the areas of BAT physiology in adult humans, developmental lineages of brown adipose cell fate, and hormonal control of BAT thermogenesis. As we enter into a new era of brown fat biology, the next challenge will be to develop strategies for activating BAT thermogenesis in adult humans to increase whole-body energy expenditure. This article reviews the recent major advances in this field and discusses emerging questions.
Figures
Figure 1
Uncoupling protein 1 (UCP1)-dependent thermogenesis in brown adipocytes. (a) Hypothetical model of UCP1-dependent proton uncoupling in brown fat mitochondria. A long-chain fatty acid (LCFA) molecule directly binds to the UCP1 protein, serving as a UCP1 substrate to transport one H+ per transport cycle. The transported free-fatty-acid (FFA) anion needs a long, hydrophobic tail. (b) ATP inhibits the activity of UCP1 in the resting state. In response to cold stimuli or excess food intake, norepinephrine acts on the β3-adrenoceptor (β3-AR), leading to the activation of cAMP-dependent protein kinase (PKA) and the phosphorylation of hormone-sensitive lipase (HSL). FFAs are generated by cAMP-induced lipolysis or taken up from the circulation and utilized as UCP1 substrates for H+ transport and as substrates of β-oxidation in brown fat mitochondria (indicated by red arrows).
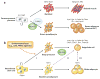
Figure 2
Hierarchical developmental relationships in brown and white adipocytes. The two types of thermogenic adipocytes (classical brown adipocytes and beige/brite cells) have separate developmental origins. (a) BAT and skeletal muscle originate from precursors in the dermomyotome that express Engrailed-1 (En1) and Myf5. Brown adipose fate in the somite is determined by transcriptional regulators, including PRDM16 (PRD1-BF-1-RIZ1 homologous domain–containing protein 16) and C/EBPβ(CCAAT/enhancer-binding protein-β), during embryonic development. (b) Beige/brite cells are not descended from _Myf5_-expressing cells. Adipocyte precursors that express PDGFα(36) differentiate into beige/brite cells mainly in subcutaneous white adipose tissue in response to several environmental cues, including chronic cold exposure, exercise, and peroxisome proliferator–activated receptor γ(PPARγ) agonists (red arrow), through the action of PRDM16. These cells appear to be derived from (i ) defined beige precursors, (ii ) directed differentiation from bipotent preadipocytes, or (iii ) transdifferentiation from mature white adipocytes. The dashed purple arrows depict hypothetical relationships that need further investigation.
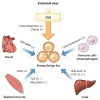
Figure 3
BAT-mediated interorgan networks. Several endocrine factors regulate BAT development and thermogenesis and mediate interorgan communication with central and peripheral tissues. Abbreviations: ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CNS, central nervous system; FGF, fibroblast growth factor; TGF, transforming growth factor.
Similar articles
- Brown adipose tissue and thermogenesis.
Fenzl A, Kiefer FW. Fenzl A, et al. Horm Mol Biol Clin Investig. 2014 Jul;19(1):25-37. doi: 10.1515/hmbci-2014-0022. Horm Mol Biol Clin Investig. 2014. PMID: 25390014 Review. - Translational Pharmacology and Physiology of Brown Adipose Tissue in Human Disease and Treatment.
Larson CJ. Larson CJ. Handb Exp Pharmacol. 2019;251:381-424. doi: 10.1007/164_2018_184. Handb Exp Pharmacol. 2019. PMID: 30689089 - Brown adipose tissue as an anti-obesity tissue in humans.
Chechi K, Nedergaard J, Richard D. Chechi K, et al. Obes Rev. 2014 Feb;15(2):92-106. doi: 10.1111/obr.12116. Epub 2013 Oct 25. Obes Rev. 2014. PMID: 24165204 Review. - Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity.
Korach-André M, Archer A, Barros RP, Parini P, Gustafsson JÅ. Korach-André M, et al. Proc Natl Acad Sci U S A. 2011 Jan 4;108(1):403-8. doi: 10.1073/pnas.1017884108. Epub 2010 Dec 20. Proc Natl Acad Sci U S A. 2011. PMID: 21173252 Free PMC article. - Brown and Beige Fat: Molecular Parts of a Thermogenic Machine.
Cohen P, Spiegelman BM. Cohen P, et al. Diabetes. 2015 Jul;64(7):2346-51. doi: 10.2337/db15-0318. Epub 2015 Jun 7. Diabetes. 2015. PMID: 26050670 Free PMC article. Review.
Cited by
- Glut4 palmitoylation at Cys223 plays a critical role in Glut4 membrane trafficking.
Ren W, Sun Y, Du K. Ren W, et al. Biochem Biophys Res Commun. 2015 May 8;460(3):709-14. doi: 10.1016/j.bbrc.2015.03.094. Epub 2015 Mar 27. Biochem Biophys Res Commun. 2015. PMID: 25824042 Free PMC article. - Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue.
Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AWC, Stasch JP, Bloch W, Friebe A, Heeren J, Pfeifer A. Hoffmann LS, et al. Nat Commun. 2015 May 26;6:7235. doi: 10.1038/ncomms8235. Nat Commun. 2015. PMID: 26011238 Free PMC article. - Genetic and functional characterization of clonally derived adult human brown adipocytes.
Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, Nedergaard J, Sidossis LS, Kajimura S. Shinoda K, et al. Nat Med. 2015 Apr;21(4):389-94. doi: 10.1038/nm.3819. Epub 2015 Mar 16. Nat Med. 2015. PMID: 25774848 Free PMC article. - Dynamic Expression Profiles of Circular RNAs during Brown to White Adipose Tissue Transformation in Goats (Capra hircus).
Zhang X, Zhan S, Yang S, Zhong T, Guo J, Cao J, Wang Y, Li L, Zhang H, Wang L. Zhang X, et al. Animals (Basel). 2021 May 10;11(5):1351. doi: 10.3390/ani11051351. Animals (Basel). 2021. PMID: 34068539 Free PMC article. - Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers.
Bargut TC, Silva-e-Silva AC, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Bargut TC, et al. Eur J Nutr. 2016 Feb;55(1):159-69. doi: 10.1007/s00394-015-0834-0. Epub 2015 Jan 23. Eur J Nutr. 2016. PMID: 25612928
References
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. - PubMed
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocr Metab. 2007;293:E444–52. - PubMed
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. - PubMed
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK097441/DK/NIDDK NIH HHS/United States
- DK087853/DK/NIDDK NIH HHS/United States
- K99 DK087853/DK/NIDDK NIH HHS/United States
- DK097441/DK/NIDDK NIH HHS/United States
- R00 DK087853/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous