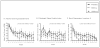Gabapentin treatment for alcohol dependence: a randomized clinical trial - PubMed (original) (raw)
Randomized Controlled Trial
Gabapentin treatment for alcohol dependence: a randomized clinical trial
Barbara J Mason et al. JAMA Intern Med. 2014 Jan.
Abstract
Importance: Approved medications for alcohol dependence are prescribed for less than 9% of US alcoholics.
Objective: To determine if gabapentin, a widely prescribed generic calcium channel/γ-aminobutyric acid-modulating medication, increases rates of sustained abstinence and no heavy drinking and decreases alcohol-related insomnia, dysphoria, and craving, in a dose-dependent manner.
Design, participants and setting: A 12-week, double-blind, placebo-controlled, randomized dose-ranging trial of 150 men and women older than 18 years with current alcohol dependence, conducted from 2004 through 2010 at a single-site, outpatient clinical research facility adjoining a general medical hospital.
Interventions: Oral gabapentin (dosages of 0 [placebo], 900 mg, or 1800 mg/d) and concomitant manual-guided counseling.
Main outcomes and measures: Rates of complete abstinence and no heavy drinking (coprimary) and changes in mood, sleep, and craving (secondary) over the 12-week study. RESULTS Gabapentin significantly improved the rates of abstinence and no heavy drinking. The abstinence rate was 4.1% (95% CI, 1.1%-13.7%) in the placebo group, 11.1% (95% CI, 5.2%-22.2%) in the 900-mg group, and 17.0% (95% CI, 8.9%-30.1%) in the 1800-mg group (P = .04 for linear dose effect; number needed to treat [NNT] = 8 for 1800 mg). The no heavy drinking rate was 22.5% (95% CI, 13.6%-37.2%) in the placebo group, 29.6% (95% CI, 19.1%-42.8%) in the 900-mg group, and 44.7% (95% CI, 31.4%-58.8%) in the 1800-mg group (P = .02 for linear dose effect; NNT = 5 for 1800 mg). Similar linear dose effects were obtained with measures of mood (F2 = 7.37; P = .001), sleep (F2 = 136; P < .001), and craving (F2 = 3.56; P = .03). There were no serious drug-related adverse events, and terminations owing to adverse events (9 of 150 participants), time in the study (mean [SD], 9.1 [3.8] weeks), and rate of study completion (85 of 150 participants) did not differ among groups.
Conclusions and relevance: Gabapentin (particularly the 1800-mg dosage) was effective in treating alcohol dependence and relapse-related symptoms of insomnia, dysphoria, and craving, with a favorable safety profile. Increased implementation of pharmacological treatment of alcohol dependence in primary care may be a major benefit of gabapentin as a treatment option for alcohol dependence.
Trial registration: clinicaltrials.gov Identifier: NCT00391716.
Figures
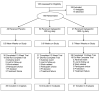
Figure 1
Flow of participants through the trial.
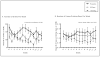
Figure 2
Gabapentin effects on rates of no heavy drinking and complete abstinence during the 12-week study in the intention-to-treat population (N = 150).
Figure 3
Gabapentin effects on number of drinks per week and number of heavy drinking days per week during the 12-week study in the intention-to-treat population (N = 150).
Figure 4
Gabapentin effects on standardized measures of craving, sleep, and mood during the 12-week study in the intention-to-treat population (N = 150).
Comment in
- Gabapentin: a new addition to the armamentarium for alcohol dependence?
Nunes EV. Nunes EV. JAMA Intern Med. 2014 Jan;174(1):78-9. doi: 10.1001/jamainternmed.2013.11973. JAMA Intern Med. 2014. PMID: 24190363 Free PMC article. No abstract available. - ACP Journal Club. Gabapentin increased complete abstinence in alcohol-dependent patients seeking treatment.
Saitz R. Saitz R. Ann Intern Med. 2014 Feb 18;160(4):JC10. doi: 10.7326/0003-4819-160-4-201402180-02010. Ann Intern Med. 2014. PMID: 24534932 No abstract available. - Gabapentin treatment for alcohol dependence.
Geisler BP, Ghosh A. Geisler BP, et al. JAMA Intern Med. 2014 Jul;174(7):1201. doi: 10.1001/jamainternmed.2014.1618. JAMA Intern Med. 2014. PMID: 25003889 No abstract available. - Gabapentin treatment for alcohol dependence--reply.
Mason BJ, Goodell V, Shadan F. Mason BJ, et al. JAMA Intern Med. 2014 Jul;174(7):1201-2. doi: 10.1001/jamainternmed.2014.1591. JAMA Intern Med. 2014. PMID: 25003890 No abstract available.
Similar articles
- Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial.
Furieri FA, Nakamura-Palacios EM. Furieri FA, et al. J Clin Psychiatry. 2007 Nov;68(11):1691-700. doi: 10.4088/jcp.v68n1108. J Clin Psychiatry. 2007. PMID: 18052562 Clinical Trial. - Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients With Alcohol Withdrawal Symptoms: A Randomized Clinical Trial.
Anton RF, Latham P, Voronin K, Book S, Hoffman M, Prisciandaro J, Bristol E. Anton RF, et al. JAMA Intern Med. 2020 May 1;180(5):728-736. doi: 10.1001/jamainternmed.2020.0249. JAMA Intern Med. 2020. PMID: 32150232 Free PMC article. Clinical Trial. - Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin.
Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Malcolm R, et al. J Clin Sleep Med. 2007 Feb 15;3(1):24-32. J Clin Sleep Med. 2007. PMID: 17557449 Clinical Trial. - The role of gabapentin in the management of alcohol withdrawal and dependence.
Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. Leung JG, et al. Ann Pharmacother. 2015 Aug;49(8):897-906. doi: 10.1177/1060028015585849. Epub 2015 May 12. Ann Pharmacother. 2015. PMID: 25969570 Review. - Gabapentin for the treatment of alcohol use disorder.
Mason BJ, Quello S, Shadan F. Mason BJ, et al. Expert Opin Investig Drugs. 2018 Jan;27(1):113-124. doi: 10.1080/13543784.2018.1417383. Epub 2017 Dec 23. Expert Opin Investig Drugs. 2018. PMID: 29241365 Free PMC article. Review.
Cited by
- Neurosteroids (allopregnanolone) and alcohol use disorder: From mechanisms to potential pharmacotherapy.
Gatta E, Camussi D, Auta J, Guidotti A, Pandey SC. Gatta E, et al. Pharmacol Ther. 2022 Dec;240:108299. doi: 10.1016/j.pharmthera.2022.108299. Epub 2022 Oct 30. Pharmacol Ther. 2022. PMID: 36323379 Free PMC article. Review. - Review article: current and emerging therapies for the management of cirrhosis and its complications.
Tapper EB, Ufere NN, Huang DQ, Loomba R. Tapper EB, et al. Aliment Pharmacol Ther. 2022 May;55(9):1099-1115. doi: 10.1111/apt.16831. Epub 2022 Mar 2. Aliment Pharmacol Ther. 2022. PMID: 35235219 Free PMC article. Review. - Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial Assessing Efficacy and Safety.
Falk DE, Ryan ML, Fertig JB, Devine EG, Cruz R, Brown ES, Burns H, Salloum IM, Newport DJ, Mendelson J, Galloway G, Kampman K, Brooks C, Green AI, Brunette MF, Rosenthal RN, Dunn KE, Strain EC, Ray L, Shoptaw S, Ait-Daoud Tiouririne N, Gunderson EW, Ransom J, Scott C, Leggio L, Caras S, Mason BJ, Litten RZ; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Falk DE, et al. Alcohol Clin Exp Res. 2019 Jan;43(1):158-169. doi: 10.1111/acer.13917. Epub 2018 Dec 9. Alcohol Clin Exp Res. 2019. PMID: 30403402 Free PMC article. Clinical Trial. - Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework.
Koob GF, Colrain IM. Koob GF, et al. Neuropsychopharmacology. 2020 Jan;45(1):141-165. doi: 10.1038/s41386-019-0446-0. Epub 2019 Jun 24. Neuropsychopharmacology. 2020. PMID: 31234199 Free PMC article. Review.
References
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet. 2009;373(9682):2223–33. - PubMed
- Substance Abuse and Mental Health Services Administration . Results from the 2006 national survey on drug use and health: national findings. Rockville, MD: 2007. [Accessed February 10, 2013]. Office of Applied Studies (NSDUH Series H-32, DHHS publication No. SMA 07-4293). http://www.samhsa.gov/data/nsduh/2k6nsduh/2k6results.pdf.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition, Text Revision. American Psychiatric Press; Washington, DC: 2000.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition. American Psychiatric Press; Washington, DC: 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous