SMTP-7, a novel small-molecule thrombolytic for ischemic stroke: a study in rodents and primates - PubMed (original) (raw)
SMTP-7, a novel small-molecule thrombolytic for ischemic stroke: a study in rodents and primates
Hironobu Sawada et al. J Cereb Blood Flow Metab. 2014 Feb.
Abstract
SMTP-7 (Stachybotrys microspora triprenyl phenol-7), a small molecule that promotes plasminogen activation through the modulation of plasminogen conformation, has excellent therapeutic activity against cerebral infarction in several rodent models. Detailed evaluations of SMTP-7 in a primate stroke model are needed for effective, safe drug development. Here we evaluated SMTP-7 in a monkey photochemical-induced thrombotic middle cerebral artery (MCA) occlusion model (n=6), in which MCA occlusion was followed by recanalization/reocclusion. SMTP-7 (10 mg/kg, intravenous infusion) significantly increased the postinfusion MCA recanalization rate (32.5-fold, P=0.043) and ameliorated the post-24-h neurologic deficit (by 29%, P=0.02), cerebral infarct (by 46%, P=0.033), and cerebral hemorrhage (by 51%, P=0.013) compared with the vehicle control animals. In normal monkeys, SMTP-7 did not affect general physiologic or hemostatic variables, including coagulation and platelet parameters. Investigations in rodent models of transient and permanent focal cerebral ischemia, as well as arterial thrombosis and bleeding tests, suggest a role for SMTP-7's regulated profibrinolytic action and neuroprotective properties in the monkey MCA occlusion model. In conclusion, SMTP-7 is effective in treating thrombotic stroke in monkeys. SMTP-7 is thus a promising candidate for the development of alternative therapy for ischemic stroke.
Figures
Figure 1
Effects of SMTP-7 (Stachybotrys microspora triprenyl phenol-7) on blood flow and neurologic deficits in a monkey thrombotic middle cerebral artery (MCA) occlusion model. (A) Blood flow profile after photochemical induction of thrombotic MCA occlusion. The experimental design and patency status of the MCA blood flow in individual animals are shown. The complete stoppage of the blood flow was regarded as occlusion and is indicated as a filled bar, and reperfusion is represented by an open bar. (B) Time for initial occlusion. (C) Postinfusion recanalization time. The sum of the duration of recanalization during the 30-minute postinfusion period is shown. (D) Neurologic deficit scores. The total of the scores was 100, comprising consciousness (range 0 to 28), sensory system (range 0 to 22), motor system (range 0 to 32), and skeletal muscle coordination (range 0 to 18). _P_-values by unpaired Student's _t_-test are shown. There were six animals in each group.
Figure 2
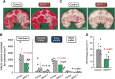
Effects of SMTP-7 (Stachybotrys microspora triprenyl phenol-7) on cerebral infarction and hemorrhage in a monkey thrombotic middle cerebral artery (MCA) occlusion model. (A) Cerebral infarction. Images are representative sections stained with TTC (2,3,5-triphenyltetrazolium chloride). Infarct areas are marked. (B) The quantification of infarction volume corrected for edema. The degree of edema was as follows: control, 25.5±2.4% SMTP-7, 20.5±2.1%. The difference was significant (_P_=0.005 by unpaired Student's _t_-test). (C) Hemorrhagic area. Images are representative nonstained sections. Arrows indicate the hemorrhage area, which is marked by a dashed circle. (D) The quantification of the hemorrhagic area. _P_-values by unpaired Student's _t_-test are shown. There were six animals in each group.
Figure 3
Effects of SMTP-7 (Stachybotrys microspora triprenyl phenol-7) on neurologic deficits and cerebral infarct in transient and permanent focal cerebral ischemia models in rats. (A and B) Neurologic deficit scores and infarction sizes corrected for edema in a transient focal ischemia model. The total of the neurologic deficit scores was 15, comprising forelimb flexion (range 0 to 3), hindlimb flexion (range 0 to 3), forelimb motor function (range 0 to 3), postural reaction after lateral pressure (range 0 to 3), and the animal's posture (range 0 to 3). The insets in A and B show the experimental design and representative TTC (2,3,5-triphenyltetrazolium chloride)-stained brain sections from vehicle-treated and SMTP-7 (10 mg/kg)-treated animals, respectively. *P<0.05 compared with vehicle control by Kruskal–Wallis test (A) or Dunnett test (B). The degree of edema was as follows: control, 15.2±3.3% 1 mg/kg, 11.4±4.4% 3 mg/kg, 12.2±3.8% 10 mg/kg, 9.8±3.0%. The difference between the control group and the 10 mg/kg group was significant (_P_=0.02 by Tukey's test). There were 10 animals in each group. (C and D) Neurologic deficit scores and infarction size corrected for edema in the permanent focal ischemia model. The definition of the neurologic deficit score is the same as that described for panel A. The inset in C shows the experimental design. There was no significant difference between the groups in neurologic score. *P<0.05 by unpaired Student's _t_-test for infarction size corrected for edema. The degree of edema was 4.2±11.3% for the control group and 12.7±6.3% for the SMTP-7 group (_P_=0.076 by unpaired Student's _t_-test). There were 10 animals in each group.
Figure 4
Effects of SMTP-7 (Stachybotrys microspora triprenyl phenol-7) on thrombotic carotid artery occlusion, bleeding, and plasmin formation in vivo. (A) Initial occlusion time in a rat carotid artery occlusion model. **P<0.01 versus vehicle control by Mann–Whitney U_-test; #P<0.05 versus control by Kruskal–Wallis test. (B) Bleeding time in normal mice. *P<0.05 versus control; **P<0.01 versus control; ##P<0.01 versus 100 mg/kg SMTP-7 group; ††_P<0.01 versus 1 mg/kg tissue-type plasminogen activator (t-PA) group by Tukey–Kramer test. There were five to eight animals in each group. (C) Plasmin–_α_2-antiplasmin complex (Pm–AP) levels in normal mice. **P<0.01 versus control by Tukey test. Insets in panels A to C show the experimental designs. There were five animals in each group. (D) In vitro analysis of the formation of Pm–AP and t-PA–plasminogen activator inhibitor-1 (PAI-1) in the presence of SMTP-7. Mouse plasma supplemented with _α_2-antiplasmin or PAI-1 was incubated with plasmin or t-PA in the presence of the indicated concentrations of SMTP-7 to assess the effects of SMTP-7 on the formation of Pm–AP and t-PA–PAI-1 in vitro. Data are representative of duplicate experiments. Positions of Pm–AP and t-PA–PAI-1 standards are shown. The extended gel images are shown in Supplementary Figure 5.
Similar articles
- Impact of SMTP Targeting Plasminogen and Soluble Epoxide Hydrolase on Thrombolysis, Inflammation, and Ischemic Stroke.
Hasumi K, Suzuki E. Hasumi K, et al. Int J Mol Sci. 2021 Jan 19;22(2):954. doi: 10.3390/ijms22020954. Int J Mol Sci. 2021. PMID: 33477998 Free PMC article. Review. - Neuroprotective effects of SMTP-44D in mice stroke model in relation to neurovascular unit and trophic coupling.
Shi X, Ohta Y, Shang J, Morihara R, Nakano Y, Fukui Y, Liu X, Feng T, Huang Y, Sato K, Takemoto M, Hishikawa N, Yamashita T, Suzuki E, Hasumi K, Abe K. Shi X, et al. J Neurosci Res. 2018 Dec;96(12):1887-1899. doi: 10.1002/jnr.24326. Epub 2018 Sep 22. J Neurosci Res. 2018. PMID: 30242877 - Reduction of Ischemia Reperfusion-Related Brain Hemorrhage by Stachybotrys Microspora Triprenyl Phenol-7 in Mice With Antioxidant Effects.
Huang Y, Ohta Y, Shang J, Li X, Liu X, Shi X, Feng T, Yamashita T, Sato K, Takemoto M, Hishikawa N, Suzuki E, Hasumi K, Abe K. Huang Y, et al. J Stroke Cerebrovasc Dis. 2018 Dec;27(12):3521-3528. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.018. Epub 2018 Sep 7. J Stroke Cerebrovasc Dis. 2018. PMID: 30201460 - Efficacy of SMTP-7, a small-molecule anti-inflammatory thrombolytic, in embolic stroke in monkeys.
Suzuki E, Nishimura N, Yoshikawa T, Kunikiyo Y, Hasegawa K, Hasumi K. Suzuki E, et al. Pharmacol Res Perspect. 2018 Dec 5;6(6):e00448. doi: 10.1002/prp2.448. eCollection 2018 Dec. Pharmacol Res Perspect. 2018. PMID: 30546909 Free PMC article. - Progress in Isoindolone Alkaloid Derivatives from Marine Microorganism: Pharmacology, Preparation, and Mechanism.
Hang S, Chen H, Wu W, Wang S, Fang Y, Sheng R, Tu Q, Guo R. Hang S, et al. Mar Drugs. 2022 Jun 20;20(6):405. doi: 10.3390/md20060405. Mar Drugs. 2022. PMID: 35736208 Free PMC article. Review.
Cited by
- Structure-activity relationships of the plasminogen modulator SMTP with respect to the inhibition of soluble epoxide hydrolase.
Matsumoto N, Suzuki E, Tsujihara K, Nishimura Y, Hasumi K. Matsumoto N, et al. J Antibiot (Tokyo). 2015 Nov;68(11):685-90. doi: 10.1038/ja.2015.58. Epub 2015 May 13. J Antibiot (Tokyo). 2015. PMID: 25966853 - A clinically relevant model of focal embolic cerebral ischemia by thrombus and thrombolysis in rhesus monkeys.
Wu D, Chen J, Wu L, Lee H, Shi J, Zhang M, Ma Y, He X, Zhu Z, Yan F, Wu C, Duan Y, Fu Y, Li S, Zhi X, Zhang X, Li S, Ding Y, Ji X. Wu D, et al. Nat Protoc. 2022 Sep;17(9):2054-2084. doi: 10.1038/s41596-022-00707-5. Epub 2022 Jun 27. Nat Protoc. 2022. PMID: 35760857 Review. - Small Molecules: Therapeutic Application in Neuropsychiatric and Neurodegenerative Disorders.
Schiavone S, Trabace L. Schiavone S, et al. Molecules. 2018 Feb 13;23(2):411. doi: 10.3390/molecules23020411. Molecules. 2018. PMID: 29438357 Free PMC article. Review. - Therapeutic Strategies to Attenuate Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment for Acute Ischemic Stroke.
Kanazawa M, Takahashi T, Nishizawa M, Shimohata T. Kanazawa M, et al. J Atheroscler Thromb. 2017 Mar 1;24(3):240-253. doi: 10.5551/jat.RV16006. Epub 2016 Dec 13. J Atheroscler Thromb. 2017. PMID: 27980241 Free PMC article. Review. - Impact of SMTP Targeting Plasminogen and Soluble Epoxide Hydrolase on Thrombolysis, Inflammation, and Ischemic Stroke.
Hasumi K, Suzuki E. Hasumi K, et al. Int J Mol Sci. 2021 Jan 19;22(2):954. doi: 10.3390/ijms22020954. Int J Mol Sci. 2021. PMID: 33477998 Free PMC article. Review.
References
- Feigin VI, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. - PubMed
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Eng J Med. 1995;333:1581–1587. - PubMed
- Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39:924–928. - PubMed
- Nadeau JO, Shi S, Fang J, Kapral MK, Richards JA, Silver FL, et al. tPA use for stroke in the Registry of the Canadian Stroke Network. Can J Neurol Sci. 2005;32:433–439. - PubMed
- Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099–1107. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical