Physical biology in cancer. 5. The rocky road of metastasis: the role of cytoskeletal mechanics in cell migratory response to 3D matrix topography - PubMed (original) (raw)
Review
Physical biology in cancer. 5. The rocky road of metastasis: the role of cytoskeletal mechanics in cell migratory response to 3D matrix topography
Francois Bordeleau et al. Am J Physiol Cell Physiol. 2014.
Abstract
The tumor microenvironment is a milieu of heterogeneous architectural features that affect tumor growth and metastatic invasion. Pore size, density, stiffness, and fiber architecture change dramatically from location to location throughout the tumor matrix. While many studies have addressed the effects of two-dimensional extracellular matrix structure and composition on cell migration, less is known about how cancer cells navigate complex, heterogeneous three-dimensional (3D) microenvironments. Mechanical structures such as actin and keratin, part of the cytoskeletal framework, and lamins, part of the nucleoskeletal framework, play a key role in migration and are altered during cancer progression. Recent evidence suggests that these changes in cytoskeletal and nucleoskeletal structures may enable cancer cells to efficiently respond to features such as pore size and stiffness to invade and migrate. Here we discuss the role of cell mechanics and the cytoskeleton in the ability of cells to navigate and respond to 3D matrix features and heterogeneities.
Keywords: actin; cytoskeleton; extracellular matrix topography; keratin; lamins; mechanosensing; nucleoskeleton; three-dimensional migration.
Figures
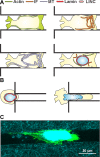
Fig. 1.
Overview of key mechanical features governing cell migration in restrictive 3-dimensional (3D) channels. A: representations of the same migrating cell inside a restrictive 3D channel showing cortical localization of actin along the wall of the channel and at both extremities of the migrating cell, intermediate filament (IF) localization at the leading edge of the migrating cell, microtubule (MT) preferential localization at the leading edge of the migrating cell, and a schematic of cytoskeletal-nucleoskeletal connections via linker of nucleoskeleton and cytoskeleton (LINC) complex coupling of lamins and actin/IFs. B: overexpression of lamin IFs stiffens the nucleus and poses a significant barrier to migration in subnuclear pore sizes, while diminished expression of lamin IFs softens the nucleus and allows for greater nuclear deformability. C: confocal image of a cell migrating inside a microfabricated 3D collagen microtrack showing actin (green) and reflectance (collagen, cyan). (See Ref. for details.)
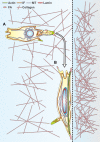
Fig. 2.
A migrating cell relies on the coordinated effort of the cytoskeleton for its mechanosensing ability in a 3D matrix. A migrating cell utilizes the polarizing features of the MT network while also relying on IF assembly and localization at the leading edge to initiate integrin binding (A). When the migrating cell reaches a stiffened interface (arrow), it has the potential to initiate localized integrin binding sites, as well as formation of stress fibers and nuclear deformation, changing the overall cell morphology to a spread shape (B).
Similar articles
- Mechanoresponsive metabolism in cancer cell migration and metastasis.
Zanotelli MR, Zhang J, Reinhart-King CA. Zanotelli MR, et al. Cell Metab. 2021 Jul 6;33(7):1307-1321. doi: 10.1016/j.cmet.2021.04.002. Epub 2021 Apr 28. Cell Metab. 2021. PMID: 33915111 Free PMC article. Review. - Physical biology in cancer. 4. Physical cues guide tumor cell adhesion and migration.
Stroka KM, Konstantopoulos K. Stroka KM, et al. Am J Physiol Cell Physiol. 2014 Jan 15;306(2):C98-C109. doi: 10.1152/ajpcell.00289.2013. Epub 2013 Oct 16. Am J Physiol Cell Physiol. 2014. PMID: 24133064 Free PMC article. Review. - Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis.
Li X, Wang J. Li X, et al. Int J Biol Sci. 2020 Apr 27;16(12):2014-2028. doi: 10.7150/ijbs.44943. eCollection 2020. Int J Biol Sci. 2020. PMID: 32549750 Free PMC article. Review. - The microenvironment and cytoskeletal remodeling in tumor cell invasion.
Azadi S, Tafazzoli Shadpour M. Azadi S, et al. Int Rev Cell Mol Biol. 2020;356:257-289. doi: 10.1016/bs.ircmb.2020.06.003. Epub 2020 Jul 4. Int Rev Cell Mol Biol. 2020. PMID: 33066875 Review. - Comparative mechanisms of cancer cell migration through 3D matrix and physiological microtracks.
Carey SP, Rahman A, Kraning-Rush CM, Romero B, Somasegar S, Torre OM, Williams RM, Reinhart-King CA. Carey SP, et al. Am J Physiol Cell Physiol. 2015 Mar 15;308(6):C436-47. doi: 10.1152/ajpcell.00225.2014. Epub 2014 Dec 10. Am J Physiol Cell Physiol. 2015. PMID: 25500742 Free PMC article.
Cited by
- Cancer cell mechanobiology: a new frontier for cancer research.
Yu W, Sharma S, Rao E, Rowat AC, Gimzewski JK, Han D, Rao J. Yu W, et al. J Natl Cancer Cent. 2021 Dec 4;2(1):10-17. doi: 10.1016/j.jncc.2021.11.007. eCollection 2022 Mar. J Natl Cancer Cent. 2021. PMID: 39035217 Free PMC article. Review. - Phenotypic Heterogeneity and Metastasis of Breast Cancer Cells.
Hapach LA, Carey SP, Schwager SC, Taufalele PV, Wang W, Mosier JA, Ortiz-Otero N, McArdle TJ, Goldblatt ZE, Lampi MC, Bordeleau F, Marshall JR, Richardson IM, Li J, King MR, Reinhart-King CA. Hapach LA, et al. Cancer Res. 2021 Jul 1;81(13):3649-3663. doi: 10.1158/0008-5472.CAN-20-1799. Epub 2021 May 11. Cancer Res. 2021. PMID: 33975882 Free PMC article. - Cancer Stem Cells: Emergent Nature of Tumor Emergency.
Efremov YR, Proskurina AS, Potter EA, Dolgova EV, Efremova OV, Taranov OS, Ostanin AA, Chernykh ER, Kolchanov NA, Bogachev SS. Efremov YR, et al. Front Genet. 2018 Nov 16;9:544. doi: 10.3389/fgene.2018.00544. eCollection 2018. Front Genet. 2018. PMID: 30505319 Free PMC article. - Matrix stiffness regulates vascular integrity through focal adhesion kinase activity.
Wang W, Lollis EM, Bordeleau F, Reinhart-King CA. Wang W, et al. FASEB J. 2019 Jan;33(1):1199-1208. doi: 10.1096/fj.201800841R. Epub 2018 Aug 13. FASEB J. 2019. PMID: 30102569 Free PMC article. - Inhibition of ovarian tumor cell invasiveness by targeting SYK in the tyrosine kinase signaling pathway.
Yu Y, Suryo Rahmanto Y, Lee MH, Wu PH, Phillip JM, Huang CH, Vitolo MI, Gaillard S, Martin SS, Wirtz D, Shih IM, Wang TL. Yu Y, et al. Oncogene. 2018 Jul;37(28):3778-3789. doi: 10.1038/s41388-018-0241-0. Epub 2018 Apr 11. Oncogene. 2018. PMID: 29643476 Free PMC article.
References
- Alam H, Kundu ST, Dalal SN, Vaidya MM. Loss of keratins 8 and 18 leads to alterations in α6β4-integrin-mediated signalling and decreased neoplastic progression in an oral-tumour-derived cell line. J Cell Sci 124: 2096–2106, 2011 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources