Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function - PubMed (original) (raw)
Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function
Wieteke A Zuure et al. J Neurosci. 2013.
Abstract
The adipocyte-derived hormone leptin acts in the brain to modulate the central driver of fertility: the gonadotropin releasing hormone (GnRH) neuronal system. This effect is indirect, as GnRH neurons do not express leptin receptors (LEPRs). Here we test whether GABAergic or glutamatergic neurons provide the intermediate pathway between the site of leptin action and the GnRH neurons. Leptin receptors were deleted from GABA and glutamate neurons using Cre-Lox transgenics, and the downstream effects on puberty onset and reproduction were examined. Both mouse lines displayed the expected increase in body weight and region-specific loss of leptin signaling in the hypothalamus. The GABA neuron-specific LEPR knock-out females and males showed significantly delayed puberty onset. Adult fertility observations revealed that these knock-out animals have decreased fecundity. In contrast, glutamate neuron-specific LEPR knock-out mice displayed normal fertility. Assessment of the estrogenic hypothalamic-pituitary-gonadal axis regulation in females showed that leptin action on GABA neurons is not necessary for estradiol-mediated suppression of tonic luteinizing hormone secretion (an indirect measure of GnRH neuron activity) but is required for regulation of a full preovulatory-like luteinizing hormone surge. In conclusion, leptin signaling in GABAergic (but not glutamatergic neurons) plays a critical role in the timing of puberty onset and is involved in fertility regulation throughout adulthood in both sexes. These results form an important step in explaining the role of central leptin signaling in the reproductive system. Limiting the leptin-to-GnRH mediators to GABAergic cells will enable future research to focus on a few specific types of neurons.
Figures
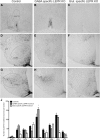
Figure 1.
Characterization of the knock-out models by labeling leptin-induced phosphorylated STAT3. A–I, Representative photomicrographs of staining in different hypothalamic areas counted. A–C, MnPO and rPOA. D–F, Arc, VMN, LH, and DMN. G–I, Arc and PMV. The first column shows leptin-induced (5 mg/kg) pSTAT3 labeling in control animals. Second and third columns show pSTAT3 labeling in GABA- and glutamate-specific LEPR knock-out animals, respectively. J, Quantification of immunohistochemical staining (number of positive labeled cells per section) in control animals (black bars; n = 11), GABA-specific (gray bars; n = 10), and glutamate-specific (white bars, n = 8; n = 2 for NTS) knock-outs. There was no difference between the pSTAT3 counts of the two control groups (n = 6 and n = 5) that are derived from the two different Cre lines; therefore, they have been pooled in this graph. In all regions, experimental groups are compared with the control animals to show significant differences. MnPO includes the region of the organum vasculosum of the lamina terminalis (OVLT); rPOA, ventral part. Scale bar, 100 μm. *p < 0.05.
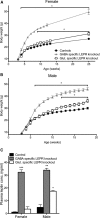
Figure 2.
Effects of LEPR knock-outs on body weight and plasma leptin concentration. A, B, Female and male GABA- and glutamate-specific LEPR knock-out and control animals body weights. Female GABA-specific knock-out animals (gray triangles; n = 10) are significantly heavier than controls (black circles; n = 19) at 4 weeks of age; glutamate-specific LEPR knock-out animals (white squares; n = 9) are significantly heavier than controls (black circles) at 9 weeks of age. Male GABA-specific LEPR knock-out animals (gray triangles; n = 9) had an increase in body weight from 6 weeks onwards and glutamate-specific knock-out males (white squares; n = 6) from 12 weeks onwards compared with controls (black circles; n = 15). We were unable to gather body weight data for the female groups throughout the breeding study because females were at different stages of pregnancy. C, Plasma leptin concentrations of control animals (black bars; female n = 8, male n = 3) are significantly lower than GABA-specific knock-out animals (gray bars; female n = 8, male n = 2). In glutamate-specific LEPR knock-outs, male animals show significantly higher leptin than the controls (white bars; female n = 4, male n = 2). *p < 0.05. **p < 0.01. ***p < 0.001.
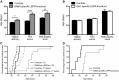
Figure 3.
Puberty onset in GABA- and glutamate-specific LEPR knock-out animals. A, In GABA-specific LEPR knock-out animals (gray bars; n = 7–10), vaginal opening, first estrus, and male puberty onset were all significantly delayed compared with their control littermates (black bars; n = 7–10). B, Vaginal opening, first estrus, and male puberty onset all occurred at the same time in glutamate-specific knock-out animals (white bars; n = 6–9) compared with their control littermates (black bars; n = 8–9). C, Survival profiles showing puberty onset of female GABA-specific LEPR knock-out (gray lines) and control animals (black lines). The percentage of mice showing vaginal opening (intermittent lines) and first estrus (continuous lines) is plotted for each time point. D, Puberty onset (date of first successful mating) profiles of male GABA-specific knock-outs (gray line) and controls (black line) over time is plotted. VO, Vaginal opening. *p < 0.05. **p < 0.01. ***p < 0.001.
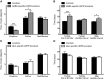
Figure 4.
Adult fertility in knock-out and control groups. A, Frequency of estrous cycle stage was monitored for 28 d in GABA-specific LEPR knock-out females (gray bars; n = 9) and controls (black bars; n = 10). A significant decrease in time spent in proestrus was seen in the knock-out females. B, The time from pairing with a wild-type male to first litter in GABA knock-out females (gray bar; n = 10) was longer than in littermate controls (black bar; n = 10). Number of days between litters was significantly increased in male GABA-specific knock-out animals (n = 7 in both groups). The same trend was seen in surviving females, but low animal number limited statistical comparison (n = 3). C, Frequency of estrous cycle stage was monitored for 10 d in glutamate-specific knock-out mice (white bars; n = 9) and control littermates (black bars; n = 9). No significant differences were seen between the groups. D, Time to first litter and number of days between litters were the same for all glutamate-specific LEPR knock-out females and males (white bars; n = 6 and 9) compared with littermate controls (black bars; n = 8 and 9). *p < 0.05. ***p < 0.001.
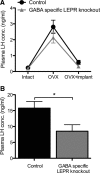
Figure 5.
The effects of estradiol on plasma LH concentration in GABA-specific LEPR knock-outs and controls. A, Estradiol-negative feedback was assessed by measuring plasma LH concentration in serial blood samples. Intact plasma samples were taken on day 0, day 14 (OVX), and day 22 (OVX+implant). The negative feedback actions of estradiol remained intact in GABA-specific LEPR knock-out animals (gray triangles; n = 9) compared with littermate controls (black circles; n = 9). B, Estradiol-positive feedback was assessed based on plasma LH concentration in trunk bloods taken at the time of an estradiol-induced preovulatory-like surge. There was a significant suppression of LH concentration in GABA-specific knock-out female animals (gray bar; n = 9) compared with controls (black bar; n = 9). *p < 0.05.
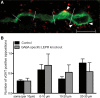
Figure 6.
GABAergic (vGAT-positive) appositions on GnRH neurons in male GABA-specific knock-outs and controls. A, Representative image of a GnRH neuron (green) with vGAT (red) appositions on the soma and proximal projection (white arrowheads). White lines indicate 10 μm segments of the neuronal projection. B, Number of vGAT-positive appositions per 10 μm of GnRH soma membrane and the first three 10 μm segments of the projection, in control (black bars; n = 5) and GABA-specific LEPR knock-out animals (gray bars; n = 5). No significant differences were found between groups. Scale bar, 10 μm.
Comment in
- Re: leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function.
Atala A. Atala A. J Urol. 2014 Jun;191(6):1928. doi: 10.1016/j.juro.2014.03.012. Epub 2014 Mar 18. J Urol. 2014. PMID: 25280328 No abstract available.
Similar articles
- Leptin Signaling in AgRP Neurons Modulates Puberty Onset and Adult Fertility in Mice.
Egan OK, Inglis MA, Anderson GM. Egan OK, et al. J Neurosci. 2017 Apr 5;37(14):3875-3886. doi: 10.1523/JNEUROSCI.3138-16.2017. Epub 2017 Mar 8. J Neurosci. 2017. PMID: 28275162 Free PMC article. - Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone.
Martin C, Navarro VM, Simavli S, Vong L, Carroll RS, Lowell BB, Kaiser UB. Martin C, et al. J Neurosci. 2014 Apr 23;34(17):6047-56. doi: 10.1523/JNEUROSCI.3003-13.2014. J Neurosci. 2014. PMID: 24760864 Free PMC article. - Expression of ESR1 in Glutamatergic and GABAergic Neurons Is Essential for Normal Puberty Onset, Estrogen Feedback, and Fertility in Female Mice.
Cheong RY, Czieselsky K, Porteous R, Herbison AE. Cheong RY, et al. J Neurosci. 2015 Oct 28;35(43):14533-43. doi: 10.1523/JNEUROSCI.1776-15.2015. J Neurosci. 2015. PMID: 26511244 Free PMC article. - Chemical identity of hypothalamic neurons engaged by leptin in reproductive control.
Ratra DV, Elias CF. Ratra DV, et al. J Chem Neuroanat. 2014 Nov;61-62:233-8. doi: 10.1016/j.jchemneu.2014.05.005. Epub 2014 Jun 7. J Chem Neuroanat. 2014. PMID: 24915437 Free PMC article. Review. - Hypothalamic sites of leptin action linking metabolism and reproduction.
Donato J Jr, Cravo RM, Frazão R, Elias CF. Donato J Jr, et al. Neuroendocrinology. 2011;93(1):9-18. doi: 10.1159/000322472. Epub 2010 Nov 24. Neuroendocrinology. 2011. PMID: 21099209 Free PMC article. Review.
Cited by
- Deletion of Suppressor of Cytokine Signaling 3 from Forebrain Neurons Delays Infertility and Onset of Hypothalamic Leptin Resistance in Response to a High Caloric Diet.
McEwen HJ, Inglis MA, Quennell JH, Grattan DR, Anderson GM. McEwen HJ, et al. J Neurosci. 2016 Jul 6;36(27):7142-53. doi: 10.1523/JNEUROSCI.2714-14.2016. J Neurosci. 2016. PMID: 27383590 Free PMC article. - Metabolic Impact on the Hypothalamic Kisspeptin-Kiss1r Signaling Pathway.
Wahab F, Atika B, Ullah F, Shahab M, Behr R. Wahab F, et al. Front Endocrinol (Lausanne). 2018 Mar 28;9:123. doi: 10.3389/fendo.2018.00123. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29643834 Free PMC article. Review. - Role of the adipocyte-derived hormone leptin in reproductive control.
Garcia-Galiano D, Allen SJ, Elias CF. Garcia-Galiano D, et al. Horm Mol Biol Clin Investig. 2014 Sep;19(3):141-9. doi: 10.1515/hmbci-2014-0017. Horm Mol Biol Clin Investig. 2014. PMID: 25390022 Free PMC article. Review. - Leptin Signaling in AgRP Neurons Modulates Puberty Onset and Adult Fertility in Mice.
Egan OK, Inglis MA, Anderson GM. Egan OK, et al. J Neurosci. 2017 Apr 5;37(14):3875-3886. doi: 10.1523/JNEUROSCI.3138-16.2017. Epub 2017 Mar 8. J Neurosci. 2017. PMID: 28275162 Free PMC article. - The ventral premammillary nucleus at the interface of environmental cues and social behaviors.
Cavalcante JC, da Silva FG, Sáenz de Miera C, Elias CF. Cavalcante JC, et al. Front Neurosci. 2025 Apr 10;19:1589156. doi: 10.3389/fnins.2025.1589156. eCollection 2025. Front Neurosci. 2025. PMID: 40276575 Free PMC article. Review.
References
- Bourguignon JP, Gerard A, Alvarez Gonzalez ML, Purnelle G, Franchimont P. Endogenous glutamate involvement in pulsatile secretion of gonadotropin-releasing hormone: evidence from effect of glutamine and developmental changes. Endocrinology. 1995;136:911–916. doi: 10.1210/en.136.3.911. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous