Cooperation between brain and islet in glucose homeostasis and diabetes - PubMed (original) (raw)
Review
Cooperation between brain and islet in glucose homeostasis and diabetes
Michael W Schwartz et al. Nature. 2013.
Abstract
Although a prominent role for the brain in glucose homeostasis was proposed by scientists in the nineteenth century, research throughout most of the twentieth century focused on evidence that the function of pancreatic islets is both necessary and sufficient to explain glucose homeostasis, and that diabetes results from defects of insulin secretion, action or both. However, insulin-independent mechanisms, referred to as 'glucose effectiveness', account for roughly 50% of overall glucose disposal, and reduced glucose effectiveness also contributes importantly to diabetes pathogenesis. Although mechanisms underlying glucose effectiveness are poorly understood, growing evidence suggests that the brain can dynamically regulate this process in ways that improve or even normalize glycaemia in rodent models of diabetes. Here we present evidence of a brain-centred glucoregulatory system (BCGS) that can lower blood glucose levels via both insulin-dependent and -independent mechanisms, and propose a model in which complex and highly coordinated interactions between the BCGS and pancreatic islets promote normal glucose homeostasis. Because activation of either regulatory system can compensate for failure of the other, defects in both may be required for diabetes to develop. Consequently, therapies that target the BCGS in addition to conventional approaches based on enhancing insulin effects may have the potential to induce diabetes remission, whereas targeting just one typically does not.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Box 1 Figure. The traditional, islet-centred model of normal and abnormal glucose homeostasis
a, Under normal conditions, the islet-centred model proposes that glucose homeostasis is controlled primarily by the effect of rising blood glucose levels to stimulate insulin secretion. Insulin then acts on peripheral tissues such as the liver to suppress hepatic glucose production (HGP), and adipose tissue and muscle to stimulate glucose uptake. Not shown is the effect of the islet hormone glucagon, secretion of which is inhibited by rising glucose levels, and which acts to stimulate HGP. Thus, glucose has opposing actions on the secretion of insulin and glucagon, hormones that in turn have opposing effects on HGP. When blood glucose levels increase (for example, during a meal), therefore, the islet response effectively returns it to baseline. b, When individuals with normal islet function become insulin-resistant (for example, in association with dietary and/or genetic factors that cause obesity), the islet-centred model proposes that glucose homeostasis is preserved by the capacity of the islet to increase insulin secretion in a compensatory manner. c, If islet dysfunction precludes the increase of insulin secretion needed to overcome insulin resistance, glucose intolerance results. As islet dysfunction progresses, increased HGP and reduced tissue glucose uptake eventually cause overt hyperglycaemia and diabetes.
Box 2 Figure. Model integrating the BCGS and islet-centred system in normal and abnormal glucose homeostasis
a, Under normal conditions, glucose homeostasis is controlled by complex and highly coordinated interactions between brain- and islet-centred systems. Like islets, the BCGS senses a variety of humoral signals, and in response to these inputs, BCGS activation increases glucose disposal by both insulin-dependent (for example, by increasing tissue insulin sensitivity) and insulin-independent (by increasing GE, which accounts for ∼50% of overall glucose disposal) mechanisms. b, Although insulin normally inhibits HGP through its direct action on the liver, an indirect pathway also exists through which insulin can preserve normal HGP and blood glucose levels even when hepatocytes cannot respond to insulin directly. We propose that this is among the effects mediated by the BCGS. c, Obesity is associated with reduced GE and with insulin resistance, and BCGS dysfunction contributes to both. When BCGS dysfunction is mild, the resulting tendency for blood glucose levels to increase stimulates insulin secretion, such that glucose homeostasis is preserved (at the expense of higher insulin levels). When BCGS dysfunction is more severe, however, even marked hyperinsulinaemia cannot preserve normal glucose homeostasis, owing in part to the inability of reduced GE to be compensated by increased insulin secretion. Thus, intact BCGS function is required for normal glucose homeostasis. d, Islet dysfunction is not compensated by BCGS activation; to the contrary, impaired islet function can itself impair BCGS function (by reducing secretion of leptin as well insulin, when islet damage is severe) creating a vicious cycle that results initially in glucose intolerance. As both BCGS and islet dysfunction progress, overt hyperglycaemia and T2D result. e, Islet dysfunction can be compensated for by supraphysiological BCGS activation, which can achieve near-normal glucose homeostasis in rodent models of diabetes via insulin-independent mechanisms. Thus, therapeutic interventions targeting the BCGS as well as the traditional islet-based system may achieve diabetes remission, whereas targeting just one system typically does not.
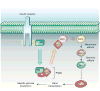
Figure 1. Insulin signal transduction
In hepatocytes, insulin regulates HGP by activating the IRS–PI(3)K pathway, which inhibits the transcription factor FOXO1. FOXO1 activation increases gluconeogenesis, leading to increased hepatic glucose production (HGP). PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-triphosphate.
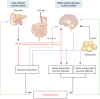
Figure 2. Schematic illustrations of brain- and islet-centred glucoregulatory systems
The BCGS is proposed to regulate tissue glucose metabolism and plasma glucose levels via mechanisms that are both insulin dependent (for example, by regulating tissue insulin sensitivity) and insulin independent. Because of extensive redundancy between islet- and brain-centred pathways, dysfunction of both may be required for T2D to develop, and diabetes remission may be possible with therapies that target both pathways.
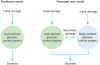
Figure 3. Proposed contributions of defective brain- and islet-centred glucoregulatory systems to T2D pathogenesis
The traditional view holds that diabetes arises as a consequence of damage to, and ultimately failure of, beta-cell function. We propose a two-component model in which failure of glucose homeostasis can begin after initial impairment of either pancreatic islets or the BCGS. Malfunction of either of the two systems can initiate a cascade that drives the remaining glucoregulatory system into failure over time. Only when both systems are compromised does diabetes develop. Consequently, interventions that target both systems have greater therapeutic potential than those that target just one system.
Similar articles
- Glucose homeostasis dependency on acini-islet-acinar (AIA) axis communication: a new possible pathophysiological hypothesis regarding diabetes mellitus.
Pierzynowski SG, Gregory PC, Filip R, Woliński J, Pierzynowska KG. Pierzynowski SG, et al. Nutr Diabetes. 2018 Oct 8;8(1):55. doi: 10.1038/s41387-018-0062-9. Nutr Diabetes. 2018. PMID: 30293998 Free PMC article. Review. - Decreasing cx36 gap junction coupling compensates for overactive KATP channels to restore insulin secretion and prevent hyperglycemia in a mouse model of neonatal diabetes.
Nguyen LM, Pozzoli M, Hraha TH, Benninger RK. Nguyen LM, et al. Diabetes. 2014 May;63(5):1685-97. doi: 10.2337/db13-1048. Epub 2014 Jan 23. Diabetes. 2014. PMID: 24458355 Free PMC article. - Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes.
Rafacho A, Ortsäter H, Nadal A, Quesada I. Rafacho A, et al. J Endocrinol. 2014 Dec;223(3):R49-62. doi: 10.1530/JOE-14-0373. Epub 2014 Sep 30. J Endocrinol. 2014. PMID: 25271217 Review. - Reducing Glucokinase Activity to Enhance Insulin Secretion: A Counterintuitive Theory to Preserve Cellular Function and Glucose Homeostasis.
Whitticar NB, Nunemaker CS. Whitticar NB, et al. Front Endocrinol (Lausanne). 2020 Jun 9;11:378. doi: 10.3389/fendo.2020.00378. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32582035 Free PMC article. - Effects of biotin deficiency on pancreatic islet morphology, insulin sensitivity and glucose homeostasis.
Larrieta E, Vega-Monroy ML, Vital P, Aguilera A, German MS, Hafidi ME, Fernandez-Mejia C. Larrieta E, et al. J Nutr Biochem. 2012 Apr;23(4):392-9. doi: 10.1016/j.jnutbio.2011.01.003. Epub 2011 May 18. J Nutr Biochem. 2012. PMID: 21596550
Cited by
- Rap1 in the VMH regulates glucose homeostasis.
Kaneko K, Lin HY, Fu Y, Saha PK, De la Puente-Gomez AB, Xu Y, Ohinata K, Chen P, Morozov A, Fukuda M. Kaneko K, et al. JCI Insight. 2021 Jun 8;6(11):e142545. doi: 10.1172/jci.insight.142545. JCI Insight. 2021. PMID: 33974562 Free PMC article. - Ketoisocaproic acid, a metabolite of leucine, suppresses insulin-stimulated glucose transport in skeletal muscle cells in a BCAT2-dependent manner.
Moghei M, Tavajohi-Fini P, Beatty B, Adegoke OA. Moghei M, et al. Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C518-27. doi: 10.1152/ajpcell.00062.2016. Epub 2016 Aug 3. Am J Physiol Cell Physiol. 2016. PMID: 27488662 Free PMC article. - Lower Glucose Effectiveness Is Associated with Postprandial Hyperglycemia in Obese/Overweight Men, Independently of Insulin Secretion.
Kishimoto I, Ohashi A. Kishimoto I, et al. Metabolites. 2022 Oct 25;12(11):1022. doi: 10.3390/metabo12111022. Metabolites. 2022. PMID: 36355105 Free PMC article. - Hyperglycemia Induced by Chronic Restraint Stress in Mice Is Associated With Nucleus Tractus Solitarius Injury and Not Just the Direct Effect of Glucocorticoids.
Zheng X, Bi W, Yang G, Zhao J, Wang J, Li X, Zhou X. Zheng X, et al. Front Neurosci. 2018 Dec 19;12:983. doi: 10.3389/fnins.2018.00983. eCollection 2018. Front Neurosci. 2018. PMID: 30618599 Free PMC article. - Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity.
Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, Eid GM, Stefanovic-Racic M, Toledo FG, Jakicic JM, Houmard JA, Goodpaster BH. Coen PM, et al. J Clin Invest. 2015 Jan;125(1):248-57. doi: 10.1172/JCI78016. Epub 2014 Dec 1. J Clin Invest. 2015. PMID: 25437877 Free PMC article. Clinical Trial.
References
- Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999– 2004. J Am Med Assoc. 2006;295:1549–1555. - PubMed
- Cowie CC, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999– 2002. Diabetes Care. 2006;29:1263–1268. - PubMed
- Bernard C. Leçons de Ohysiologie Experimentale Appliqués á lá Medecine. Paris: J.-B. Baillière; 1854.
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. - PubMed
- Morton GJ, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. http://dx.doi.org/10.1172/JCI70710 (1 October 2013) Administration of a low dose of the hormone FGF19 directly into the brain of leptin-deficient ob/ob mice ameliorated glucose intolerance by rapidly, potently and selectively increasing glucose effectiveness. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK090320/DK/NIDDK NIH HHS/United States
- DK093848/DK/NIDDK NIH HHS/United States
- R01 DK089056/DK/NIDDK NIH HHS/United States
- R01 DK083042/DK/NIDDK NIH HHS/United States
- R01 DK093848/DK/NIDDK NIH HHS/United States
- DK089053/DK/NIDDK NIH HHS/United States
- P30 DK035816/DK/NIDDK NIH HHS/United States
- DK083042/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical