Microenvironmental regulation of tumor progression and metastasis - PubMed (original) (raw)
Review
Microenvironmental regulation of tumor progression and metastasis
Daniela F Quail et al. Nat Med. 2013 Nov.
Abstract
Cancers develop in complex tissue environments, which they depend on for sustained growth, invasion and metastasis. Unlike tumor cells, stromal cell types within the tumor microenvironment (TME) are genetically stable and thus represent an attractive therapeutic target with reduced risk of resistance and tumor recurrence. However, specifically disrupting the pro-tumorigenic TME is a challenging undertaking, as the TME has diverse capacities to induce both beneficial and adverse consequences for tumorigenesis. Furthermore, many studies have shown that the microenvironment is capable of normalizing tumor cells, suggesting that re-education of stromal cells, rather than targeted ablation per se, may be an effective strategy for treating cancer. Here we discuss the paradoxical roles of the TME during specific stages of cancer progression and metastasis, as well as recent therapeutic attempts to re-educate stromal cells within the TME to have anti-tumorigenic effects.
Figures
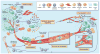
Figure 1. Multiple stromal cell types converge to support a tumorigenic primary niche
After circumventing cell-intrinsic mechanisms of apoptosis, tumor cells are subject to elimination pressures by the immune system. Tumor cell-specific antigens play a role during this process, which are recognized by cytotoxic immune cells, leading to their destruction. Fibroblasts and macrophages within the TME also contribute to a growth-suppressive state; however, these cells may later become educated by the tumor to acquire pro-tumorigenic functions. For instance, tumor-associated macrophages (TAMs) support diverse phenotypes within the primary tumor including growth, angiogenesis and invasion by secreting a plethora of pro-tumorigenic proteases, cytokines and growth factors (e.g. EGF, which participates in a paracrine signaling loop via tumor-secreted CSF-1). As tumors grow, immune-suppressor cells, including myeloid-derived suppressor cells (MDSCs) and Treg cells are mobilized into circulation in response to activated cytokine axes induced by tumorigenesis (e.g. TGF-β, CXCL5-CXCR2). MDSCs and Treg cells infiltrate the growing tumor to disrupt immune surveillance via multiple mechanisms, including, but not limited to, disruption of antigen presentation by DCs, inhibition of T and B cell proliferation and activation, or inhibition of NK cytotoxicity. Cancer-associated fibroblasts (CAFs), which become activated by tumor-derived factors (e.g. TGF-β, FGF, PDGF, etc), secrete ECM proteins and basement membrane components, regulate differentiation, modulate immune responses, and contribute to deregulated homeostasis. CAFs are also a key source of VEGF, which supports angiogenesis during tumor growth. In addition to cellular contributions, several extracellular properties contribute to tumor progression, including low oxygen tension, high interstitial fluid pressure, and changes in specific components of the ECM.
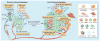
Figure 2. The microenvironment supports metastatic dissemination and colonization at secondary sites
Macrophages, platelets, and mesenchymal stem cells (MSCs) contribute to epithelial-to-mesenchymal transition (EMT) at primary sites, allowing for tumor cells to separate from neighboring epithelial cell-cell contacts and acquire a mobile/invasive phenotype. One major mediator of this event is TGF-β, which is secreted by the tumor stroma and participates in a paracrine signaling loop with tumor cells. TAMs, CAFs and myeloid progenitor cells also tend to cluster at the invasive/leading edge of the primary tumor, where they play an immunosuppressive role by interfering with dendritic cell differentiation. During intravasation of tumor cells into circulation, intravital imaging studies have shown that macrophages are localized to perivascular areas within tumors, where they help tumor cells traverse vessel barriers. In the circulation, platelets and components of the coagulation system support tumor cell survival by protecting them from cytotoxic immune cell recognition. Platelets escort tumor cells in circulation to the site of extravasation, where they bind to areas of vascular retraction and help tumor cells exit circulation into secondary organs. At secondary sites such as the lung, fibroblasts upregulate fibronectin, which serves as a docking site for hematopoietic progenitor cells (HPCs) and the subsequent arrival of tumor cells. Immunosuppressive cell types, such as MDSCs and NK cells, also populate premetastatic niches where they help to direct metastatic dissemination by creating a niche permissive to tumor colonization. Recent studies have demonstrated that primary and secondary sites can communicate through exosomes, shed not only by primary tumor cells but also by immune and stromal cells such as NK cells, CAFs and dendritic cells. Factors contained in exosomes have the capacity to direct organ tropism, modulate immune evasion, support mesenchymal-to-epithelial transition (MET), and are predictive of metastasis and patient outcome.
Figure 3. Overcoming tumor dormancy, and initiation of secondary outgrowth in metastatic niches
Dormant micrometastases are held in check by several mechanisms. Tumor mass dormancy, or angiogenic dormancy, is when proliferation is balanced by apoptosis, owing to a lack of vasculature and limited supply of nutrients and oxygen. Multiple cell types contribute to the re-establishment of vascularity at the secondary site, including hematopoietic and endothelial progenitor cells (HPCs; EPCs) expressing VEGF receptors, and dendritic cell precursors which can differentiate into an endothelial-like state. Tumor cells can also exist in a state of cellular dormancy, whereby proliferation is arrested in G0. This can be overcome via several mechanisms, for example, fibronectin-integrin interactions and activation of EGFR signaling, or re-polarization of macrophages from an anti- to a pro-tumorigenic state within the TME. Last, tumor cells can enter immune-induced dormancy whereby immunogenic cells are cleared, and cells that are able to survive enter a state of equilibrium. Immune suppressor cells are recruited to tumors in response to this process, and contribute to the establishment of an immunosuppressive state within secondary tissues. Treg cells and MDSCs are depicted here, which produce anti-inflammatory cytokines and suppress the anti-tumorigenic capacities of other immune cell types. Once micrometastases overcome dormancy, they become receptive to signals and cell types within their microenvironment to further support their expansion. For instance, TAMs are abundant in metastases of multiple cancer types, and support different tumorigenic processes to allow for outgrowth, including vascularization, impaired immunogenicity, and enhanced survival in overt metastases. Platelets, and components of the coagulation system, such as tissue factor (TF), are also important mediators of metastatic outgrowth, as they interfere with the ability of NK cells to destroy micrometastases, and support clot formation, which in turn causes the recruitment of MDSCs.
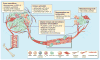
Figure 4. Therapeutic strategies to re-educate the tumor microenvironment
Multiple strategies to target the TME are either currently in clinical use, or at different stages of clinical development, as indicated here, and referenced throughout the review. The tumor vasculature can be targeted with multiple drugs, such as bevacizumab (targets VEGF-A), CXCR2 antagonists, Sunitinib (a multi-target RTK inhibitor), and VEGF-Trap (soluble decoy receptor for VEGF). Immune activation, marked by an induction of T cells, is also a promising avenue of therapeutic intervention. This can be achieved through blockade of CTLA-4 (ipilimumab), PD1 receptor (nivolumab), or PD1 Ligand (lambrolizumab). Repolarization or re-education of cells within the TME, in particular macrophages or other myeloid cells, can be achieved by CSF-1R inhibition (e.g. BLZ945), or monoclonal antibodies that activate CD40. Alternatively, immune cell recruitment and expansion can be blocked through inhibition of critical cytokine axes, such as CXCR4 (AMD3100), CXCR2 (S-265610), CSF-1R and/or KIT (PLX3397), and the chemotherapeutic agent trabectedin, whose anti-tumor activity is proposed to be a result of selective depletion of monocytes/macrophages. Likewise, metastatic seeding and outgrowth can be blocked by inhibition of key cytokine axes, such as CCR2 (MLN1202).
Similar articles
- [Role of the stroma in the initiation and progression of tumors].
Baranyi M, Lippai M, Szatmári Z. Baranyi M, et al. Orv Hetil. 2015 Nov 8;156(45):1816-23. doi: 10.1556/650.2015.30294. Orv Hetil. 2015. PMID: 26522855 Review. Hungarian. - Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site.
Nasrollahzadeh E, Razi S, Keshavarz-Fathi M, Mazzone M, Rezaei N. Nasrollahzadeh E, et al. Cancer Immunol Immunother. 2020 Sep;69(9):1673-1697. doi: 10.1007/s00262-020-02616-6. Epub 2020 Jun 4. Cancer Immunol Immunother. 2020. PMID: 32500231 Free PMC article. Review. - Research progress of tumor microenvironment and tumor-associated macrophages.
Liang W, Huang X, Carlos CJJ, Lu X. Liang W, et al. Clin Transl Oncol. 2020 Dec;22(12):2141-2152. doi: 10.1007/s12094-020-02367-x. Epub 2020 May 23. Clin Transl Oncol. 2020. PMID: 32447645 Review. - How Cells Communicate with Each Other in the Tumor Microenvironment: Suggestions to Design Novel Therapeutic Strategies in Cancer Disease.
Zefferino R, Piccoli C, Di Gioia S, Capitanio N, Conese M. Zefferino R, et al. Int J Mol Sci. 2021 Mar 4;22(5):2550. doi: 10.3390/ijms22052550. Int J Mol Sci. 2021. PMID: 33806300 Free PMC article. Review.
Cited by
- Deep learning-based automatic image classification of oral cancer cells acquiring chemoresistance in vitro.
Hsieh HC, Chen CY, Chou CH, Peng BY, Sun YC, Lin TW, Chien Y, Chiou SH, Hung KF, Lu HH. Hsieh HC, et al. PLoS One. 2024 Nov 1;19(11):e0310304. doi: 10.1371/journal.pone.0310304. eCollection 2024. PLoS One. 2024. PMID: 39485749 Free PMC article. - Editorial: Understanding the mesenchymal to epithelial transition: a much needed angle for epithelial mesenchymal plasticity.
Alotaibi H, Meuwissen R, Sayan AE. Alotaibi H, et al. Front Cell Dev Biol. 2024 Oct 7;12:1497515. doi: 10.3389/fcell.2024.1497515. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39435331 Free PMC article. No abstract available. - Identification and immunoprofiling of key prognostic genes in the tumor microenvironment of hepatocellular carcinoma.
Wang T, Chen B, Meng T, Liu Z, Wu W. Wang T, et al. Bioengineered. 2021 Dec;12(1):1555-1575. doi: 10.1080/21655979.2021.1918538. Bioengineered. 2021. PMID: 33955820 Free PMC article. - Clinical utility of TGFB1 and its receptors (TGFBR1 and TGFBR2) in thyroid nodules: evaluation based on single nucleotide polymorphisms and mRNA analysis.
Peres KC, Teodoro L, Amaral LHP, Teixeira ES, Barreto IS, de Freitas LLL, Maximo V, Assumpção LVM, Bufalo NE, Ward LS. Peres KC, et al. Arch Endocrinol Metab. 2021 Nov 1;65(2):172-184. doi: 10.20945/2359-3997000000330. Epub 2021 Feb 24. Arch Endocrinol Metab. 2021. PMID: 33905626 Free PMC article. - Imaging of tumor clones with differential liver colonization.
Oshima G, Wightman SC, Uppal A, Stack ME, Pitroda SP, Oskvarek JJ, Huang X, Posner MC, Hellman S, Weichselbaum RR, Khodarev NN. Oshima G, et al. Sci Rep. 2015 Jun 22;5:10946. doi: 10.1038/srep10946. Sci Rep. 2015. PMID: 26094901 Free PMC article.
References
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. - PubMed
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine. 2011;17:1359–1370. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources