Phenotypic variants of staphylococci and their underlying population distributions following exposure to stress - PubMed (original) (raw)
Phenotypic variants of staphylococci and their underlying population distributions following exposure to stress
Laura A Onyango et al. PLoS One. 2013.
Abstract
This study investigated whether alterations in environmental conditions would induce the formation of small colony variant phenotypes (SCV) with associated changes in cell morphology and ultra-structure in S. aureus, s. epidermidis, and S. lugdunensis. Wild-type clinical isolates were exposed to low temperature (4 °C), antibiotic stress (penicillin G and vancomycin; 0-10,000 µg mL(-1)), pH stress (pH 3-9) and osmotic challenge (NaCl concentrations of 0-20%). Changes in cell diameter, cell-wall thickness, and population distribution changes (n ≥ 300) were assessed via scanning and transmission electron microscopy (SEM and TEM), and compared to control populations. Our analyses found that prolonged exposure to all treatments resulted in the subsequent formation of SCV phenotypes. Observed SCVs manifested as minute colonies with reduced haemolysis and pigmentation (NaCl, pH and 4°C treatments), or complete lack thereof (antibiotic treatments). SEM comparison analyses revealed significantly smaller cell sizes for SCV populations except in S. aureus and S. epidermidis 10% NaCl, and S. epidermidis 4 °C (p<0.05). Shifts in population distribution patterns were also observed with distinct sub-populations of smaller cells appearing for S. epidermidis, and S. lugdunensis. TEM analyses revealed significantly thicker cell-walls in all treatments and species except S. lugdunensis exposed to 4 °C. These findings suggest that staphylococci adapted to environmental stresses by altering their cell size and wall thickness which could represent the formation of altered phenotypes which facilitate survival under harsh conditions. The phenotypic response was governed by the type of prevailing environmental stress regime leading to appropriate alterations in ultra-structure and size, suggesting downstream changes in gene expression, the proteome, and metabolome.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
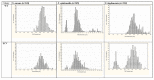
Figure 1. Population distributions of cell sizes from WT colony and corresponding SCV colony cells (4°C) of S. aureus, S. epidermidis and S. lugdunensis which have been ranked on the basis of their cell diameter measurements following SEM analyses.
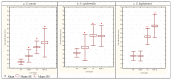
Figure 2. Mean cell sizes assessed by SEM (n>300) of WT cells and corresponding SCV cells of S. aureus (a), S. epidermidis (b) and S. lugdunensis (c).
SCV cells were generated following exposure to the antibiotics penicillin G (Pen G) and vancomycin (VA) (random antibiotic concentrations generating SCV were used), 10% NaCl, 4°C temperature and pH 5 stresses. Asterisk (*) indicates significant differences compared with corresponding WT cells (P<0.05).
Figure 3. SEM images of S. aureus, S. epidermidis and S. lugdunensis WT and their vancomycin (VA) -induced (100 µg mL-1) SCVs: SEM images with SCV cells displaying a more prevalent extracellular matrix material (arrow) than their corresponding WT cells.
Figure 4. Comparisons of mean cell-wall thickness (nanometres) from SCV cells generated following exposures to 4°C and antibiotics (VA and Pen G; random concentrations utilised) in comparison with their corresponding WT cells taken from S. aureus, S. epidermidis, and S. lugdunensis samples and examined under TEM (n=300).
Asterisk (*) indicates significant differences compared with corresponding WT cells (P<0.05).
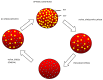
Figure 5. Diagram scheme indicating possible stages of wild-type-SCV life/stress cycle.
Under optimal conditions, WT populations prevail, perhaps masking the SCV phenotype. Introduction of stress selects for a more resilient phenotype, changing the population dynamics from WT prevalence to SCV prevalence which persist even under prolonged exposure to stress. Removal of stress shifts population dynamics with WT populations dominating again.
Similar articles
- Effect of low temperature on growth and ultra-structure of Staphylococcus spp.
Onyango LA, Dunstan RH, Gottfries J, von Eiff C, Roberts TK. Onyango LA, et al. PLoS One. 2012;7(1):e29031. doi: 10.1371/journal.pone.0029031. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22291884 Free PMC article. - Combinatorial efficacy of Manuka honey and antibiotics in the in vitro control of staphylococci and their small colony variants.
Liang J, Adeleye M, Onyango LA. Liang J, et al. Front Cell Infect Microbiol. 2023 Oct 19;13:1219984. doi: 10.3389/fcimb.2023.1219984. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37928190 Free PMC article. - Small changes in environmental parameters lead to alterations in antibiotic resistance, cell morphology and membrane fatty acid composition in Staphylococcus lugdunensis.
Crompton MJ, Dunstan RH, Macdonald MM, Gottfries J, von Eiff C, Roberts TK. Crompton MJ, et al. PLoS One. 2014 Apr 8;9(4):e92296. doi: 10.1371/journal.pone.0092296. eCollection 2014. PLoS One. 2014. PMID: 24714666 Free PMC article. - Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds.
Yong YY, Dykes GA, Choo WS. Yong YY, et al. Crit Rev Microbiol. 2019 Mar;45(2):201-222. doi: 10.1080/1040841X.2019.1573802. Epub 2019 Feb 20. Crit Rev Microbiol. 2019. PMID: 30786799 Review.
Cited by
- Changes in Amino Acid Metabolism of Staphylococcus aureus following Growth to the Stationary Phase under Adjusted Growth Conditions.
Alreshidi M, Dunstan H, Roberts T, Bardakci F, Badraoui R, Adnan M, Saeed M, Alreshidi F, Albulaihed Y, Snoussi M. Alreshidi M, et al. Microorganisms. 2022 Jul 25;10(8):1503. doi: 10.3390/microorganisms10081503. Microorganisms. 2022. PMID: 35893561 Free PMC article. - Phenotypic and Genotypic Characteristics of Small Colony Variants and Their Role in Chronic Infection.
Johns BE, Purdy KJ, Tucker NP, Maddocks SE. Johns BE, et al. Microbiol Insights. 2015 Sep 22;8:15-23. doi: 10.4137/MBI.S25800. eCollection 2015. Microbiol Insights. 2015. PMID: 26448688 Free PMC article. Review. - The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study.
Hackemann VCJ, Hagel S, Jandt KD, Rödel J, Löffler B, Tuchscherr L. Hackemann VCJ, et al. Int J Mol Sci. 2023 Nov 14;24(22):16308. doi: 10.3390/ijms242216308. Int J Mol Sci. 2023. PMID: 38003500 Free PMC article. - Imaging studies of bacterial biofilms on cochlear implants-Bioactive glass (BAG) inhibits mature biofilm.
Kirchhoff L, Arweiler-Harbeck D, Arnolds J, Hussain T, Hansen S, Bertram R, Buer J, Lang S, Steinmann J, Höing B. Kirchhoff L, et al. PLoS One. 2020 Feb 21;15(2):e0229198. doi: 10.1371/journal.pone.0229198. eCollection 2020. PLoS One. 2020. PMID: 32084198 Free PMC article. - Mechanisms of Antibiotic Failure During Staphylococcus aureus Osteomyelitis.
Gimza BD, Cassat JE. Gimza BD, et al. Front Immunol. 2021 Feb 12;12:638085. doi: 10.3389/fimmu.2021.638085. eCollection 2021. Front Immunol. 2021. PMID: 33643322 Free PMC article. Review.
References
- Almquist E (1922) Variation and life cycles of pathogenic bacteria. J Infect Dis 31: 483-493. doi:10.1093/infdis/31.5.483. - DOI
- Hadley P (1926) The instability of bacterial species with special reference to active dissociation and transmissible autolysis. J Infect Dis 40: 4465-4762.
- Onyango LA, Dunstan RH, Roberts TK (2008) Small colony variants of staphylococci: Pathogenesis and evolutionary significance in causing and sustaining problematic human infections. J Nutr Environ Med 17: 56-75. doi:10.1080/13590840801887272. - DOI
- Prescott LM, Harley JP, Klein DA (2002) Microbial growth - The influence of environmental factors on growth. Microbiology. 5th ed.. NY: McGraw-Hill.
Publication types
MeSH terms
Substances
Grants and funding
This work was partially supported via a University of Newcastle International Scholarship and Gideon Lang Scholarship, and additional funding from the Harold Stannet Williams and Judith Mason Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources