Lin28 enhances tissue repair by reprogramming cellular metabolism - PubMed (original) (raw)
Lin28 enhances tissue repair by reprogramming cellular metabolism
Ng Shyh-Chang et al. Cell. 2013.
Abstract
Regeneration capacity declines with age, but why juvenile organisms show enhanced tissue repair remains unexplained. Lin28a, a highly conserved RNA-binding protein expressed during embryogenesis, plays roles in development, pluripotency, and metabolism. To determine whether Lin28a might influence tissue repair in adults, we engineered the reactivation of Lin28a expression in several models of tissue injury. Lin28a reactivation improved hair regrowth by promoting anagen in hair follicles and accelerated regrowth of cartilage, bone, and mesenchyme after ear and digit injuries. Lin28a inhibits let-7 microRNA biogenesis; however, let-7 repression was necessary but insufficient to enhance repair. Lin28a bound to and enhanced the translation of mRNAs for several metabolic enzymes, thereby increasing glycolysis and oxidative phosphorylation (OxPhos). Lin28a-mediated enhancement of tissue repair was negated by OxPhos inhibition, whereas a pharmacologically induced increase in OxPhos enhanced repair. Thus, Lin28a enhances tissue repair in some adult tissues by reprogramming cellular bioenergetics. PAPERCLIP:
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
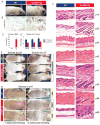
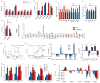
Figure 1. Lin28a reactivation promotes hair regrowth
A. Uninduced iLin28a Tg mice possess a thicker fur coat than WT mice. B. Lin28a expression in the skin of WT or iLin28a Tg mice as determined by immunohistochemistry. C. Lin28a mRNA levels as determined by qRT-PCR. D. Mature let-7 expression as determined by qRT-PCR in tail epidermis of WT and uninduced iLin28a Tg mice. E. Hair regrowth in mice shaved at p21 was observed 1 week post-shaving in 0/6 WT vs. 6/6 Lin28a Tg littermates (Left image). Hair regrowth in mice shaved at p70 was observed 3 weeks post-shaving in 0/6 WT vs. 5/5 iLin28a Tg littermates (Right image). F. Histologic hair cycle analysis over 10 weeks. All sections are 10x and H+E stained. G. Hair regrowth on dorsal skin in topical dox-treated p47 WT and iLin28a Tg littermates, 1 and 2 weeks after shaving. Ectopic hair regrowth was observed in 0/4 WT dox treated vs. 0/3 DMSO treated Tg vs. 3/4 dox induced Tg mice. Data are represented as mean +/− SEM, * P<0.05, ** P< 0.01. See also Figure S1.
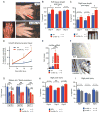
Figure 2. Lin28a reactivation promotes regrowth of digits after neonatal amputation
For all digit measurements, % regrowth is quantified by dividing the length of the regrowing digit in the injured limb with the length of the normal digit on the opposite uninjured limb. A. Left image shows p1 digits and the amputation site (blue hash mark). Right images show digits 21 days after neonatal digit amputation. Red bar depicts the soft tissue dimension measured between the digit tip and the proximal interphalangeal joint. B. Quantification of soft tissue regrowth 21 days after neonatal digit amputation. C. Quantification of bone regrowth 21 days after neonatal digit amputation. X-ray film with red bar showing that bone length was measured from the proximal interphalangeal joint. D. Post-injury growth kinetics (as % of uninjured digit length) over 21 days. E. Lin28a mRNA expression in WT, injured WT, iLin28a Tg, and injured iLin28a Tg digits, as determined by qRT-PCR. F. Immunohistochemistry indicating Lin28a protein expression in Tg and WT bone from the neonatal skeleton. G. Mature let-7 expression in WT, injured WT, iLin28a Tg, and injured iLin28a Tg digits, as determined by qRT-PCR. H. Digit tip regrowth in iLin28a Tg mice backcrossed onto the hyper-regenerative MRL strain background. Control mice are WT MRL littermates. Data are represented as mean +/− SEM, * P<0.05, ** P< 0.01. See also Figure S2.
Figure 3. Lin28a reactivation promotes pinnal tissue repair
A. WT and iLin28a Tg wound healing 8 days after 2mm-diameter ear hole punches. B. Wound area size at day 5, 8, and 11, during the course of wound healing. C. Proportion of WT and iLin28a Tg ear holes torn by mice after ear hole punching. D. Western blot indicating Lin28a protein levels in topical dox-treated iLin28a Tg ears, before (n=2) and 3 days after (n=2) injury, compared to WT ears. E. mRNA levels of Lin28a and the let-7 target Hmga2 in topical dox-treated iLin28a Tg ears, as determined by qRT-PCR. F. Wound area size after 10 days of local topical treatment with dox. G. H+E, trichrome, Lin28a, and Ki-67 staining in WT, iLin28a Tg and induced iLin28a Tg mice. Data are represented as mean +/− SEM, * P<0.05, ** P< 0.01. See also Figure S3.
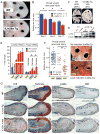
Figure 4. Let-7 repression is necessary but insufficient for tissue repair
A. Expression of mature let-7g and let-7b in WT and iLet-7 ears, before and after injury, as determined by qRT-PCR. B. Ear hole wound area size after whole animal let-7g induction in iLet-7 mice. C. Mature let-7 miRNA expression in MEFs after let-7 antimiR treatment, as determined by qRT-PCR. D. Mature let-7 miRNA expression in vivo after 2 subcutaneous injections of let-7 antimiR. E. Northern blot indicating let-7a levels in the liver and skin of control and let-7 antimiR-treated WT mice. F. Ear hole wound size after subcutaneous let-7 antimiR treatment of WT mice. G. Ear hole wound size after local topical let-7 antimiR treatment of WT mice. H. Hair regrowth after let-7 antimiR treatment of WT mice. Data are represented as mean +/− SEM, * P<0.05, ** P< 0.01.
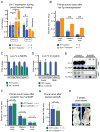
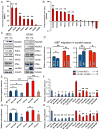
Figure 5. Lin28a alters the bioenergetic state during tissue repair
A. LC-MS/MS selected reaction monitoring (SRM) analysis of abundance in glycolysis intermediates in WT and iLin28a Tg pinnal tissue after injury (inj), relative to WT uninjured pinnae. G3P, D-glyceraldehyde-3-phosphate. DHAP, dihydroxyacetone-phosphate. BPG, 1,3-bisphosphoglycerate. 3PG, 3-phosphoglycerate. PEP, phosphoenolpyruvate. B. SRM analysis of several metabolic indicators in WT and iLin28a Tg pinnal tissue after injury (inj), relative to WT uninjured pinnae. ATP, adenosine-5′-triphosphate. AMP, adenosine-5′-monophosphate. GTP, guanosine-5′-triphosphate. GMP, guanosine-5′-monophosphate. GSH/GSSG, glutathione/glutathione disulfide. C. Fraction of glycolytic intermediates labeled by 13C, derived from [U-13C]glucose in MEFs over 30 minutes, as measured by SRM analysis (n=3). D. Fraction of Krebs cycle intermediates labeled at 2 carbons by 13C, derived from [U- 13 C]glucose in MEFs over 8 hours, as measured by SRM analysis (n=3). E. SRM analysis of the ATP/AMP and GSH/GSSG ratios in WT and iLin28a Tg MEFs (n=3). F. Mitochondrial biogenesis, glycolytic enzyme, and let-7 target mRNAs, analyzed by qRT-PCR. Lin28a, and the let-7 targets Imp1 and Imp2 served as positive controls. Relative expression levels were normalized to WT MEFs. G. Oxygen consumption rate (OCR) of WT and iLin28a Tg MEFs, as measured by the Seahorse Analyzer (n=4 each). Oligomycin treatment inhibits ATP synthase-dependent OCR, the proton gradient uncoupler FCCP then induces maximal OCR, and antimycin/rotenone finally inhibits all OxPhos-dependent OCR. H. Extracellular acidification rate (ECAR) of WT and iLin28a Tg MEFs, as measured by the Seahorse Analyzer (n=4 each). Addition of glucose induces glycolysis-dependent lactic acid production and ECAR, oligomycin then induces maximal ECAR, and 3BP partially inhibits glycolysis-dependent ECAR. I. Mature let-7 expression in WT and iLin28a Tg MEFs, after transfection with a scrambled (scr) control, let-7 LNA antimiR, or _let-7_a duplex, as determined by qRT-PCR. J. Expression of the let-7 targets Hmga2 and Imp2 in WT and iLin28a Tg MEFs, after transfection with a scrambled (scr) control, let-7 LNA antimiR, or _let-7_a duplex, as determined by qRT-PCR. K. Fraction of the glycolytic intermediate 3-phosphoglycerate (3PG) and the glycolytic side-product serine, labeled by 13C derived from [U-13C]glucose over 30 minutes, in MEFs after transfection with a scrambled (scr) control, let-7 LNA antimiR, or _let-7_a duplex (n=3). L. Fraction of the Krebs cycle intermediates citrate, malate and oxaloacetate-derived aspartate, labeled at 2 carbons by 13C derived from [U-13C]glucose over 8 hours, in MEFs after transfection with a scrambled (scr) control, let-7 LNA antimiR, or _let-7_a duplex (n=3). M. SRM analysis of several metabolic indicators in WT and iLin28a Tg MEFs after transfection with a scrambled (scr) control, let-7 LNA antimiR, or _let-7_a duplex (n=3). Data are represented as mean +/− SEM, * P<0.05, ** P< 0.01. See also Figure S5.
Figure 6. Lin28 promotes wound healing in vitro by enhancing bioenergetic metabolism
A. RNA immunoprecipitation (RIP) using FLAG-tagged Lin28a and subsequent RT-PCR shows the metabolic enzyme mRNAs bound by Lin28a in MEFs in vitro. B. RNA immunoprecipitation (RIP) using FLAG-tagged Lin28a and subsequent RT-PCR shows the metabolic enzyme mRNAs bound by Lin28a in pinnal tissues in vivo. C. Western blots for Lin28a mRNA targets in primary MEFs and pinnal tissues in vivo, D. Distance traveled by WT and iLin28a Tg MEFs, 18 hours after a defined scratch was made on equal-numbered monolayers (n=18). MEFs were treated with 50nM Anti-A, 100uM 3BP, or DMSO vehicle control immediately after the scratch. E. Distance traveled by WT and iLin28a Tg MEFs treated with a scrambled (scr) control, let-7 LNA antimiR, or _let-7_a duplex. F. Distance traveled by WT and iLin28a Tg MEFs treated with siRNAs against metabolic enzymes. G. Cell proliferation of WT and iLin28a Tg MEFs treated with a scrambled (scr) control, let-7 LNA antimiR, or _let-7_a duplex. H. Cell proliferation of WT and iLin28a Tg MEFs treated with siRNAs against metabolic enzymes. Data are represented as mean +/− SEM, * P<0.05, ** P< 0.01. See also Figure S6.
Figure 7. Lin28 promotes tissue repair in vivo by enhancing bioenergetic metabolism
A. Hair regrowth of dorsal skin in p42 iLin28a Tg mice and WT littermates, at the time of shaving, and 3 weeks after local topical treatment with 3BP, or Anti-A, dissolved in 1g/L dox in DMSO (Ctrl). Mice were then taken off all treatment for 1 week. B. Pinnal wound size after 10 days of local topical treatment with Anti-A, dissolved in 1g/L dox in DMSO (Ctrl), on both iLin28a Tg and WT littermate ear holes. C. Pinnal wound size after 10 days of local topical treatment with the antioxidant N-acetyl-cysteine (NAC) dissolved in 1g/L dox in DMSO (Ctrl), on both iLin28a Tg and WT littermate ear holes. D. Pinnal wound size after 10 days of local topical treatment with the glycolysis inhibitors 3BP or 2-deoxy-D-glucose (2DG) dissolved in 1g/L dox in DMSO (Ctrl), on both iLin28a Tg and WT littermate ear holes. E. Metabolomic profiling on WT ears 1 and 7 days after daily 3BP treatment. Shown are glycolytic intermediates and the bioenergetics ratios NADH/NAD, ATP/AMP, ATP/ADP, and GTP/GMP (n=4). F. Fraction of the Krebs cycle intermediates isocitrate, succinate, and malate, labeled at 2 carbons by 13C derived from [U-13C]glucose over 8 hours, in WT MEFs after continuous incubation in 3BP for 3 days (n=3). G. Maximal O2 consumption rate in WT MEFs after continuous incubation in 3BP for 3 days (n=6). Data are represented as mean +/− SEM, * P<0.05, ** P< 0.01.
Comment in
- Lin28: time for tissue repair.
Reddien PW. Reddien PW. Cell. 2013 Nov 7;155(4):738-9. doi: 10.1016/j.cell.2013.10.025. Cell. 2013. PMID: 24209612 - Metabolism: Young again with Lin28.
Wrighton KH. Wrighton KH. Nat Rev Mol Cell Biol. 2014 Jan;15(1):4. doi: 10.1038/nrm3715. Epub 2013 Nov 27. Nat Rev Mol Cell Biol. 2014. PMID: 24281190 No abstract available. - Lin28a--boost your energy for youthful regeneration.
Burkhalter MD, Morita Y, Rudolph KL. Burkhalter MD, et al. EMBO J. 2014 Jan 7;33(1):5-6. doi: 10.1002/embj.201387363. Epub 2013 Dec 19. EMBO J. 2014. PMID: 24357531 Free PMC article.
Similar articles
- Inhibition of Let-7b-5p maturation by LIN28A promotes thermal skin damage repair after burn injury.
Zhou S, Guo L, Cui X, Zhang X, Yang Y, Zhang M, Zhang P. Zhou S, et al. Cell Signal. 2024 Aug;120:111217. doi: 10.1016/j.cellsig.2024.111217. Epub 2024 May 8. Cell Signal. 2024. PMID: 38729326 - Lin28a expression protects against streptozotocin-induced β-cell destruction and prevents diabetes in mice.
Sung Y, Jeong J, Kang RJ, Choi M, Park S, Kwon W, Lee J, Jang S, Park SJ, Kim SH, Yi J, Choi SK, Lee MH, Liu K, Dong Z, Ryoo ZY, Kim MO. Sung Y, et al. Cell Biochem Funct. 2019 Apr;37(3):139-147. doi: 10.1002/cbf.3376. Epub 2019 Mar 18. Cell Biochem Funct. 2019. PMID: 30883865 - RNA-Binding Protein LIN28a Regulates New Myocyte Formation in the Heart Through Long Noncoding RNA-H19.
Rigaud VOC, Hoy RC, Kurian J, Zarka C, Behanan M, Brosious I, Pennise J, Patel T, Wang T, Johnson J, Kraus LM, Mohsin S, Houser SR, Khan M. Rigaud VOC, et al. Circulation. 2023 Jan 24;147(4):324-337. doi: 10.1161/CIRCULATIONAHA.122.059346. Epub 2022 Oct 31. Circulation. 2023. PMID: 36314132 Free PMC article. - The role of Lin28A and Lin28B in cancer beyond Let-7.
Cotino-Nájera S, García-Villa E, Cruz-Rosales S, Gariglio P, Díaz-Chávez J. Cotino-Nájera S, et al. FEBS Lett. 2024 Dec;598(24):2963-2979. doi: 10.1002/1873-3468.15004. Epub 2024 Aug 16. FEBS Lett. 2024. PMID: 39152528 Free PMC article. Review. - Lin28/let-7 axis in breast cancer.
Shaik Syed Ali P, Ahmad MP, Parveen KMH. Shaik Syed Ali P, et al. Mol Biol Rep. 2025 Mar 14;52(1):311. doi: 10.1007/s11033-025-10413-6. Mol Biol Rep. 2025. PMID: 40085362 Review.
Cited by
- miRNA control of tissue repair and regeneration.
Sen CK, Ghatak S. Sen CK, et al. Am J Pathol. 2015 Oct;185(10):2629-40. doi: 10.1016/j.ajpath.2015.04.001. Epub 2015 Jun 6. Am J Pathol. 2015. PMID: 26056933 Free PMC article. Review. - Simulated Microgravity Exerts an Age-Dependent Effect on the Differentiation of Cardiovascular Progenitors Isolated from the Human Heart.
Fuentes TI, Appleby N, Raya M, Bailey L, Hasaniya N, Stodieck L, Kearns-Jonker M. Fuentes TI, et al. PLoS One. 2015 Jul 10;10(7):e0132378. doi: 10.1371/journal.pone.0132378. eCollection 2015. PLoS One. 2015. PMID: 26161778 Free PMC article. - Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming.
Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Soufi A, et al. Cell. 2015 Apr 23;161(3):555-568. doi: 10.1016/j.cell.2015.03.017. Epub 2015 Apr 16. Cell. 2015. PMID: 25892221 Free PMC article. - A novel and safe small molecule enhances hair follicle regeneration by facilitating metabolic reprogramming.
Son MJ, Jeong JK, Kwon Y, Ryu JS, Mun SJ, Kim HJ, Kim SW, Yoo S, Kook J, Lee H, Kim J, Chung KS. Son MJ, et al. Exp Mol Med. 2018 Dec 6;50(12):1-15. doi: 10.1038/s12276-018-0185-z. Exp Mol Med. 2018. PMID: 30523246 Free PMC article. - Chemical reprogramming of human somatic cells to pluripotent stem cells.
Guan J, Wang G, Wang J, Zhang Z, Fu Y, Cheng L, Meng G, Lyu Y, Zhu J, Li Y, Wang Y, Liuyang S, Liu B, Yang Z, He H, Zhong X, Chen Q, Zhang X, Sun S, Lai W, Shi Y, Liu L, Wang L, Li C, Lu S, Deng H. Guan J, et al. Nature. 2022 May;605(7909):325-331. doi: 10.1038/s41586-022-04593-5. Epub 2022 Apr 13. Nature. 2022. PMID: 35418683
References
- Adams DS, et al. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;1335:1323–1335. - PubMed
- Ambros V, Horvitz H. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. - PubMed
- Anderson SP, et al. Delayed liver regeneration in peroxisome proliferator-activated receptor-α-null mice. Hepatology. 2002;36:544–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials