Enzymatically driven transport: a kinetic theory for nuclear export - PubMed (original) (raw)
Enzymatically driven transport: a kinetic theory for nuclear export
Sanghyun Kim et al. Biophys J. 2013.
Abstract
Nuclear import and export are often considered inverse processes whereby transport receptors ferry protein cargo through the nuclear pore. In contrast to import, where the reversible binding of receptor to nuclear RanGTP leads to a balanced bidirectional exchange, termination of export by physiologically irreversible hydrolysis of the Ran-bound GTP leads to unidirectional transport. We present a concise mathematical model that predicts protein distributions and kinetic rates for receptor-mediated nuclear export, which further exhibit an unexpected pseudolinear relation one to the other. Predictions of the model are verified with permeabilized and live cell measurements.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
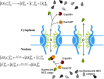
Schematic representation of nuclear export. Exportin E binds cooperatively to RanGTP, R T, and NES-cargo, C e, to form the trimeric transport complex, E R T C e. This complex is disrupted by hydrolysis of Ran-bound GTP to RanGDP, R D. The nuclear pore permeabilities are p for the cargo alone, and a for the cargo in complex with exportin and RanGTP. In the text and equations, R refers to RanGTP only.
Figure 2
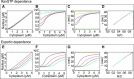
Nuclear export properties calculated by kinetic modeling. Graphs from left to right show steady-state nuclear versus cytoplasmic concentration, steady-state nuclear/cytoplasmic concentration ratio, time constant of predicted fluorescence recovery as a function of cytoplasmic concentration, and time constant of predicted fluorescence recovery plotted against the nuclear/cytoplasmic ratio. (A_–_D) Predictions for a series of RanGTP concentrations, properly the ratio of concentration to affinity: [R]SSN/KER = 1 (black), 2/10 (red), 5/100 (blue), and 1/100 (green), with total nuclear exportin concentration ESSN = 0.1 _μ_M. (E_–_H) The same predictions for a series of exportin concentrations: ESSN = 0.1 (black), 0.2 (red), 0.3 (blue), and 0.4 _μ_M (green) with [R]SSN/KER = 1. The common parameters used in calculation were v N = 800 × 10−18_μ_m3, v C = 4000 × 10−18_μ_m3, a = 100 × 10−18_μ_m3/s; p = 15 × 10−18_μ_m3/s, k e = 10 × 107 M−1 s−1, k e' = 200 × 10−2 s−1, and K e = k e '/k e = 20 nM.
Figure 3
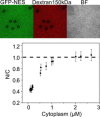
Steady-state in permeabilized cells. (Upper) GFP-NES distribution, TRITC-dextran exclusion, and bright-field images of a typical field of cells. (Lower) Steady-state N/C ratio as a function of the GFP-NES cytosolic concentration. Note the saturation of N/C at both ends of the curve: to 1 for cargo concentrations >1 _μ_M, and to 0.4 for concentrations <∼0.25 _μ_M.
Figure 4
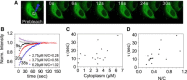
FRAP measures exchange kinetics in permeabilized cells with GFP-NES concentrations as marked: 0.18 _μ_M, 0.54 _μ_M, 0.76 _μ_M, and 2.66 _μ_M. At each concentration, approximately two to four FRAP measurements were performed in different nuclei. Since the flux is balanced at steady state, a measurement of fluorescence recovery in the nucleus is equivalent to measurement of the export kinetics. (A) Recovery images. (B) Recovery curves for various cytoplasmic GFP-NES cargo concentrations. Note the strong dependence of recovery rate with concentration. At the highest concentration, almost identical FRAP behavior was observed, indicating saturation of the kinetics to the passive flux. (C) Time constants as a function of cytoplasmic cargo concentration. (D) Time constants as a function of observed nuclear/cytoplasmic ratio.
Figure 5
FRAP in live cells expressing exogenous GFP-NES (17 cells), with presentation similar to that in Fig. 4 above.
Similar articles
- Nuclear-cytoplasmic trafficking of NTF2, the nuclear import receptor for the RanGTPase, is subjected to regulation.
Chafe SC, Pierce JB, Mangroo D. Chafe SC, et al. PLoS One. 2012;7(8):e42501. doi: 10.1371/journal.pone.0042501. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880006 Free PMC article. - Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation.
Görlich D, Seewald MJ, Ribbeck K. Görlich D, et al. EMBO J. 2003 Mar 3;22(5):1088-100. doi: 10.1093/emboj/cdg113. EMBO J. 2003. PMID: 12606574 Free PMC article. - Dissecting in vivo steady-state dynamics of karyopherin-dependent nuclear transport.
Lolodi O, Yamazaki H, Otsuka S, Kumeta M, Yoshimura SH. Lolodi O, et al. Mol Biol Cell. 2016 Jan 1;27(1):167-76. doi: 10.1091/mbc.E15-08-0601. Epub 2015 Nov 4. Mol Biol Cell. 2016. PMID: 26538027 Free PMC article. - Ran-dependent nuclear export mediators: a structural perspective.
Güttler T, Görlich D. Güttler T, et al. EMBO J. 2011 Aug 31;30(17):3457-74. doi: 10.1038/emboj.2011.287. EMBO J. 2011. PMID: 21878989 Free PMC article. Review. - Regulation of nuclear import and export by the GTPase Ran.
Steggerda SM, Paschal BM. Steggerda SM, et al. Int Rev Cytol. 2002;217:41-91. doi: 10.1016/s0074-7696(02)17012-4. Int Rev Cytol. 2002. PMID: 12019565 Review.
Cited by
- Simple rules for passive diffusion through the nuclear pore complex.
Timney BL, Raveh B, Mironska R, Trivedi JM, Kim SJ, Russel D, Wente SR, Sali A, Rout MP. Timney BL, et al. J Cell Biol. 2016 Oct 10;215(1):57-76. doi: 10.1083/jcb.201601004. Epub 2016 Oct 3. J Cell Biol. 2016. PMID: 27697925 Free PMC article. - Competition between histone and transcription factor binding regulates the onset of transcription in zebrafish embryos.
Joseph SR, Pálfy M, Hilbert L, Kumar M, Karschau J, Zaburdaev V, Shevchenko A, Vastenhouw NL. Joseph SR, et al. Elife. 2017 Apr 20;6:e23326. doi: 10.7554/eLife.23326. Elife. 2017. PMID: 28425915 Free PMC article. - Enhanced Nucleocytoplasmic Transport due to Competition for Elastic Binding Sites.
Fogelson B, Keener JP. Fogelson B, et al. Biophys J. 2018 Jul 3;115(1):108-116. doi: 10.1016/j.bpj.2018.05.034. Biophys J. 2018. PMID: 29972802 Free PMC article. - Molecular determinants of large cargo transport into the nucleus.
Paci G, Zheng T, Caria J, Zilman A, Lemke EA. Paci G, et al. Elife. 2020 Jul 21;9:e55963. doi: 10.7554/eLife.55963. Elife. 2020. PMID: 32692309 Free PMC article. - Thermodynamic Paradigm for Solution Demixing Inspired by Nuclear Transport in Living Cells.
Wang CH, Mehta P, Elbaum M. Wang CH, et al. Phys Rev Lett. 2017 Apr 14;118(15):158101. doi: 10.1103/PhysRevLett.118.158101. Epub 2017 Apr 10. Phys Rev Lett. 2017. PMID: 28452496 Free PMC article.
References
- Pemberton L.F., Paschal B.M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. - PubMed
- Terry L.J., Shows E.B., Wente S.R. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous