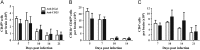Role of CD25(+) CD4(+) T cells in acute and persistent coronavirus infection of the central nervous system - PubMed (original) (raw)
Role of CD25(+) CD4(+) T cells in acute and persistent coronavirus infection of the central nervous system
Maria Teresa P de Aquino et al. Virology. 2013 Dec.
Abstract
The influence of CD25(+)CD4(+) regulatory T cells (Treg) on acute and chronic viral infection of the central nervous system (CNS) was examined using a glial tropic murine coronavirus. Treg in the CNS were highest during initial T cell mediated virus control, decreased and then remained relatively stable during persistence. Anti-CD25 treatment did not affect CNS recruitment of inflammatory cells. Viral control was initially delayed; however, neither the kinetics of viral control nor viral persistence were affected. By contrast, the absence of Treg during the acute phase resulted in increased demyelination during viral persistence. These data suggest that CNS inflammation, progression of viral control and viral persistence are relatively independent of CD25(+)CD4(+) Treg. However, their absence during acute infection alters the ability of the host to limit tissue damage.
Keywords: Acute encephalitis; Coronavirus; Demyelination; Regulatory T cells; Viral persistence.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
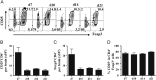
Fig. 1
Recruitment of CD25+ Treg into the CNS. CD25+Foxp3+CD4+ T cells in the CNS of infected mice analyzed by flow cytometry. (A) Representative density plots of Foxp3 and CD25 expression, gated on CD4+ T cells. Numbers represent percentages of each population. Bar graphs depict total CD25+ (B) and total Foxp3+ CD4+ T cells (C) recruited into the infected CNS. (D) Frequency of CD25+ within CD4+ Foxp3+ T cells. Data represent mean ±SEM of 6–9 individual mice per time point from at least 2 separate experiments.
Fig. 2
Kinetics of Nrp-1hi and Nrp-1low CD4+Foxp3+ in the infected CNS. Neuropilin-1 (Nrp-1) expression on CD4+Foxp3+ T cells within the infected CNS analyzed by flow cytometry. (A) Representative density plots of Nrp1 expression. Gated on CNS derived CD45hiFoxp3+CD4+ T cells. Numbers represent percentages of each population. Bar graphs depicting frequencies of Nrp-1hi cells in CD4+Foxp3+ T cells (B), CD25+ cells in Nrp-1hiFoxp3+CD4+ T cells (C) and CD25+ in Nrp-1lowFoxp3+CD4+ T cells (D). Data represent mean ±SEM of 3–6 individual mice per time point.
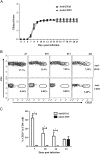
Fig. 3
Encephalomyelitis is independent of CD25+CD4+ T cells. WT mice received 250 μg of anti-CD25 or isotype control mAb i.p. at days −3, 0 and 3 relative to JHMV infection. (A) Clinical symptoms of anti-CD25 treated and control mice. Data are mean±SEM and represent 3 combined experiments comprising 53 mice per group. (B) Flow cytometric analysis of CD25 expression on CD45hiCD4+ T cells isolated from the infected CNS. Circled populations represent percentages of CD25+ T cells within CD4+ T cells. Representative data from 2 separate experiments. (C) Bar graph representing the frequency of CD25+ T cells within the CNS. Data are mean±SD and represent a combination of 2–7 experiments with 3 mice per time point in each experiment.
Fig. 4
Recruitment of CNS inflammatory cells in the absence of CD25+ T cells. CNS inflammatory cells in infected anti-CD25 or control treated mice. Bar graphs depict total CD45hi inflammatory cells (A), CD11b+CD45hi macrophages (B) and CD4+ T cells (C) per brain of infected mice. Data are mean±SD of 2–3 combined experiments with pooled brains from 3 mice per time point in each experiment.
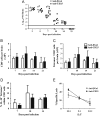
Fig. 5
Viral control is independent of CD25+CD4+ T cells. (A) Infectious virus within the CNS of individual anti-CD25 and control mAb treated mice. Data represent 2 combined experiments. Line represents the limit of detection. Statistical differences determined by two tailed unpaired t test. *_p_=<0.05. Bar graphs depict total CD8+ T cells (B) and virus specific CD8+ T cells (C) per brain. (D) Frequencies of IL-10+tetramer+ cells within in CNS CD8+ T cells. Data are mean±SD and represent 2–4 combined experiments with 3 mice per time point in each experiment. (E) Virus specific cytolytic activity of CNS derived mononuclear cells from mice with or without anti-CD25 treatment. Data are expressed as percentage of specific lysis (mean±SEM) of S510 peptide coated targets at various effector: target (E:T) ratios, adjusted for the frequency of tetramer+CD8+ T cells. Data are representative of 2 separate experiments with pooled cells from 6–7 mice per group.
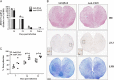
Fig. 6
CD25+ T cell influence on JHMV persistence and pathology. (A) Relative transcript levels of viral N protein mRNA in spinal cords during persistence. Data expressed as mean±SEM relative to GAPDH mRNA with 3 individuals per time point. Representative of 2 experiments with the same trend in each experiment. (B) Spinal cords from JHMV infected WT mice treated with either anti-CD25 or anti-βGal mAb at day 14 p.i. Inflammatory cells identified with H&E (top panel), viral infected cells identified with anti-N mAb J3.3 (middle panel) and demyelination assessed with LFB (bottom panel). Representative sections from 2 experiments with 3–5 mice per experiment. Bar=200 μm. Insert bars=50 μm. (C) Percentage demyelination in spinal cord white matter in individual mice calculated by analysis of transverse sections at 6 separate levels per mouse expressed as mean±SEM with 3–5 mice per time point. Representative of 2 separate experiments with the same trends. Statistical differences determined by two tailed unpaired t test. *_p_=<0.05.
Similar articles
- Regulatory T cells selectively preserve immune privilege of self-antigens during viral central nervous system infection.
Cervantes-Barragán L, Firner S, Bechmann I, Waisman A, Lahl K, Sparwasser T, Thiel V, Ludewig B. Cervantes-Barragán L, et al. J Immunol. 2012 Apr 15;188(8):3678-85. doi: 10.4049/jimmunol.1102422. Epub 2012 Mar 9. J Immunol. 2012. PMID: 22407917 - Single-Cell RNA Sequencing Reveals the Diversity of the Immunological Landscape following Central Nervous System Infection by a Murine Coronavirus.
Syage AR, Ekiz HA, Skinner DD, Stone C, O'Connell RM, Lane TE. Syage AR, et al. J Virol. 2020 Nov 23;94(24):e01295-20. doi: 10.1128/JVI.01295-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999036 Free PMC article. - CD4(+)CD25(+) T regulatory cells inhibit CD8(+) IFN-gamma production during acute and chronic FIV infection utilizing a membrane TGF-beta-dependent mechanism.
Fogle JE, Mexas AM, Tompkins WA, Tompkins MB. Fogle JE, et al. AIDS Res Hum Retroviruses. 2010 Feb;26(2):201-16. doi: 10.1089/aid.2009.0162. AIDS Res Hum Retroviruses. 2010. PMID: 20156102 Free PMC article. - MHV infection of the CNS: mechanisms of immune-mediated control.
Marten NW, Stohlman SA, Bergmann CC. Marten NW, et al. Viral Immunol. 2001;14(1):1-18. doi: 10.1089/08828240151061329. Viral Immunol. 2001. PMID: 11270593 Review. - Regulatory CD4(+)CD25(+) T-cells are controlled by multiple pathways at multiple levels.
Qu Y, Zhao Y. Qu Y, et al. Int Rev Immunol. 2007 May-Aug;26(3-4):145-60. doi: 10.1080/08830180701365917. Int Rev Immunol. 2007. PMID: 17558742 Review.
Cited by
- Roles of regulatory T cells and IL-10 in virus-induced demyelination.
Perlman S, Zhao J. Perlman S, et al. J Neuroimmunol. 2017 Jul 15;308:6-11. doi: 10.1016/j.jneuroim.2017.01.001. Epub 2017 Jan 4. J Neuroimmunol. 2017. PMID: 28065579 Free PMC article. Review. - Differential Regulation of Self-reactive CD4+ T Cells in Cervical Lymph Nodes and Central Nervous System during Viral Encephalomyelitis.
Savarin C, Bergmann CC, Hinton DR, Stohlman SA. Savarin C, et al. Front Immunol. 2016 Sep 21;7:370. doi: 10.3389/fimmu.2016.00370. eCollection 2016. Front Immunol. 2016. PMID: 27708643 Free PMC article. - IL-27 limits central nervous system viral clearance by promoting IL-10 and enhances demyelination.
de Aquino MT, Kapil P, Hinton DR, Phares TW, Puntambekar SS, Savarin C, Bergmann CC, Stohlman SA. de Aquino MT, et al. J Immunol. 2014 Jul 1;193(1):285-94. doi: 10.4049/jimmunol.1400058. Epub 2014 Jun 2. J Immunol. 2014. PMID: 24890725 Free PMC article. - Probable Neuropsychological and Cognitive Complications Due to Cytokine Storm in Patients With COVID-19.
Keshtgar Z, Chalabianloo G, Esmaeili N. Keshtgar Z, et al. Basic Clin Neurosci. 2023 Sep-Oct;14(5):549-564. doi: 10.32598/bcn.2022.3202.1. Epub 2023 Sep 1. Basic Clin Neurosci. 2023. PMID: 38628831 Free PMC article. Review. - How to detect and track chronic neurologic sequelae of COVID-19? Use of auditory brainstem responses and neuroimaging for long-term patient follow-up.
Ogier M, Andéol G, Sagui E, Dal Bo G. Ogier M, et al. Brain Behav Immun Health. 2020 May;5:100081. doi: 10.1016/j.bbih.2020.100081. Epub 2020 May 15. Brain Behav Immun Health. 2020. PMID: 32427134 Free PMC article. Review.
References
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 2007;7:875–888. - PubMed
- Bergmann C.C., Altman J.D., Hinton D., Stohlman S.A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1999;163:3379–3387. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials