Tolerance and exhaustion: defining mechanisms of T cell dysfunction - PubMed (original) (raw)
Review
Tolerance and exhaustion: defining mechanisms of T cell dysfunction
Andrea Schietinger et al. Trends Immunol. 2014 Feb.
Abstract
CD8 T cell activation and differentiation are tightly controlled, and dependent on the context in which naïve T cells encounter antigen, can either result in functional memory or T cell dysfunction, including exhaustion, tolerance, anergy, or senescence. With the identification of phenotypic and functional traits shared in different settings of T cell dysfunction, distinctions between such dysfunctional states have become blurred. Here, we discuss distinct states of CD8 T cell dysfunction, with an emphasis on: (i) T cell tolerance to self-antigens (self-tolerance); (ii) T cell exhaustion during chronic infections; and (iii) tumor-induced T cell dysfunction. We highlight recent findings on cellular and molecular characteristics defining these states, cell-intrinsic regulatory mechanisms that induce and maintain them, and strategies that can lead to their reversal.
Keywords: CD8 T cells; T cell differentiation; T cell dysfunction; chronic infection; exhaustion; self-tolerance; tumors.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
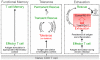
Figure 1
T cell differentiation of naïve CD8 T cells results in either functional T cell memory or T cell dysfunction as reflected by self-tolerance or exhaustion in chronic infections.
Functional memory:
When naïve CD8 T cells encounter (foreign) antigen in a stimulatory and inflammatory context (e.g. acute infection), T cells differentiate into effector and eventually into memory T cells.
Tolerance:
Peripheral self-reactive CD8 T cells that encounter self-antigen in a tolerogenic context acquire a program of functional unresponsiveness. Tolerant T cells can be transiently rescued by inducing cell proliferation, e.g. by cytokines (IL-2, IL-15) or lymphopenia. However, once proliferation stops, rescued self-reactive T cells are re-tolerized. If self-tolerant T cells can be permanently reprogrammed and rescued remains to be determined.
Exhaustion:
Virus-specific T cells initially acquire some effector functions early during chronic infections, but, due to persistence of viral antigen and inflammation, T cells become progressively exhausted. Exhausted T cells represent a heterogeneous T cell population containing T-bethiPD-1int and EomeshiPD-1hi subpopulations (see text). T-bethiPD-1int but not EomeshiPD-1hi exhausted T cells can be functionally rescued by PD-1 blockade.
Figure 2
Factors mediating exhaustion in chronic infections and tumor-induced T cell dysfunction. Exhausted virus-specific CD8 T cells in chronic infections (left) and dysfunctional tumor-infiltrating CD8 T cells (TIL, right) exhibit an `exhausted' state induced by high viral or tumor antigen load and immunosuppressive factors. `Exhausted' T cells in both settings share phenotypic and functional characteristics, including the expression of inhibitory receptors such as PD-1, LAG-3, 2B4, TIM-3, CTLA-4. However, TIL can represent a heterogeneous cell population with distinct individual `states' of dysfunction. Such dysfunctional states can be mediated by cell-intrinsic programs, including a tolerance program imprinted in self/tumor antigen-specific T cells, or programs induced in tumor-specific T cells that encounter tumor antigen early during a pre-malignant non-inflammatory phase of tumor development (red). Consequently, depending on the nature of the T cells and the microenvironment in which they must function, TIL might require different immunotherapeutic strategies to restore cell function. Abbreviations: MDSC = myeloid-derived suppressor cells; Treg = regulatory CD4 T cells; TGF-β = transforming growth factor β; NOS = Nitric Oxide Synthase; ROS = reactive oxygen species.
Similar articles
- Infection of adult thymus with murine retrovirus induces virus-specific central tolerance that prevents functional memory CD8+ T cell differentiation.
Takamura S, Kajiwara E, Tsuji-Kawahara S, Masumoto T, Fujisawa M, Kato M, Chikaishi T, Kawasaki Y, Kinoshita S, Itoi M, Sakaguchi N, Miyazawa M. Takamura S, et al. PLoS Pathog. 2014 Mar 20;10(3):e1003937. doi: 10.1371/journal.ppat.1003937. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24651250 Free PMC article. - Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections.
Philip M, Schietinger A. Philip M, et al. Curr Opin Immunol. 2019 Jun;58:98-103. doi: 10.1016/j.coi.2019.04.014. Epub 2019 Jun 8. Curr Opin Immunol. 2019. PMID: 31181510 Free PMC article. Review. - Molecular signature of CD8+ T cell exhaustion during chronic viral infection.
Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Wherry EJ, et al. Immunity. 2007 Oct;27(4):670-84. doi: 10.1016/j.immuni.2007.09.006. Epub 2007 Oct 18. Immunity. 2007. PMID: 17950003 - Peripheral tolerance in CD8+ T cells.
Srinivasan M, Frauwirth KA. Srinivasan M, et al. Cytokine. 2009 May;46(2):147-59. doi: 10.1016/j.cyto.2009.01.010. Epub 2009 Mar 5. Cytokine. 2009. PMID: 19268604 Review. - Distinct mechanisms mediate naive and memory CD8 T-cell tolerance.
Jellison ER, Turner MJ, Blair DA, Lingenheld EG, Zu L, Puddington L, Lefrançois L. Jellison ER, et al. Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21438-43. doi: 10.1073/pnas.1217409110. Epub 2012 Dec 10. Proc Natl Acad Sci U S A. 2012. PMID: 23236165 Free PMC article.
Cited by
- Exploring the prognostic impact of absolute lymphocyte count in patients treated with immune-checkpoint inhibitors.
Conroy MR, O'Sullivan H, Collins DC, Bambury RM, Power D, Grossman S, O'Reilly S. Conroy MR, et al. BJC Rep. 2024 Apr 18;2(1):31. doi: 10.1038/s44276-024-00058-6. BJC Rep. 2024. PMID: 39516713 Free PMC article. - Cardiovascular adverse events and immune-related adverse events associated with PD-1/PD-L1 inhibitors for head and neck squamous cell carcinoma (HNSCC).
Abulizi A, Yan G, Xu Q, Muhetaer R, Wu S, Abudukelimu K, Chen X, Liu C, Li J. Abulizi A, et al. Sci Rep. 2024 Oct 29;14(1):25919. doi: 10.1038/s41598-024-75099-5. Sci Rep. 2024. PMID: 39472591 Free PMC article. - Role of T Lymphocytes in Glioma Immune Microenvironment: Two Sides of a Coin.
Noor L, Upadhyay A, Joshi V. Noor L, et al. Biology (Basel). 2024 Oct 21;13(10):846. doi: 10.3390/biology13100846. Biology (Basel). 2024. PMID: 39452154 Free PMC article. Review. - Lymphocyte activation gene 3 served as a potential prognostic and immunological biomarker across various cancer types: a clinical and pan-cancer analysis.
Liu Y, Yao Y, Yang X, Wei M, Lu B, Dong K, Lyu D, Li Y, Guan W, Huang R, Xu G, Pan X. Liu Y, et al. Clin Transl Immunology. 2024 Oct 4;13(10):e70009. doi: 10.1002/cti2.70009. eCollection 2024. Clin Transl Immunology. 2024. PMID: 39372371 Free PMC article. - The miRNA and PD-1/PD-L1 signaling axis: an arsenal of immunotherapeutic targets against lung cancer.
Yadav R, Khatkar R, Yap KC, Kang CY, Lyu J, Singh RK, Mandal S, Mohanta A, Lam HY, Okina E, Kumar RR, Uttam V, Sharma U, Jain M, Prakash H, Tuli HS, Kumar AP, Jain A. Yadav R, et al. Cell Death Discov. 2024 Sep 29;10(1):414. doi: 10.1038/s41420-024-02182-1. Cell Death Discov. 2024. PMID: 39343796 Free PMC article. Review.
References
- Kaech SM, et al. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–51. - PubMed
- Baitsch L, et al. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33:364–72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials