Massively parallel polymerase cloning and genome sequencing of single cells using nanoliter microwells - PubMed (original) (raw)
. 2013 Dec;31(12):1126-32.
doi: 10.1038/nbt.2720. Epub 2013 Nov 10.
Affiliations
- PMID: 24213699
- PMCID: PMC3875318
- DOI: 10.1038/nbt.2720
Massively parallel polymerase cloning and genome sequencing of single cells using nanoliter microwells
Jeff Gole et al. Nat Biotechnol. 2013 Dec.
Abstract
Genome sequencing of single cells has a variety of applications, including characterizing difficult-to-culture microorganisms and identifying somatic mutations in single cells from mammalian tissues. A major hurdle in this process is the bias in amplifying the genetic material from a single cell, a procedure known as polymerase cloning. Here we describe the microwell displacement amplification system (MIDAS), a massively parallel polymerase cloning method in which single cells are randomly distributed into hundreds to thousands of nanoliter wells and their genetic material is simultaneously amplified for shotgun sequencing. MIDAS reduces amplification bias because polymerase cloning occurs in physically separated, nanoliter-scale reactors, facilitating the de novo assembly of near-complete microbial genomes from single Escherichia coli cells. In addition, MIDAS allowed us to detect single-copy number changes in primary human adult neurons at 1- to 2-Mb resolution. MIDAS can potentially further the characterization of genomic diversity in many heterogeneous cell populations.
Figures
Figure 1
Microwell displacement amplification system. (a) Each slide contains 16 arrays of 255 microwells each. Cells, lysis solution, denaturing buffer, neutralization buffer and MDA master mix were each added to the microwells with a single pipette pump. Amplicon growth was then visualized with a fluorescent microscope using a real-time MDA system. Microwells showing increasing fluorescence over time were positive amplicons. The amplicons were extracted with fine glass pipettes attached to a micromanipulation system. (b) Scanning electron microscopy of a single E. coli cell displayed at different magnifications. This particular well contains only one cell, and most wells observed also contained no more than one cell. (c) A custom microscope incubation chamber was used for real time MDA. The chamber was temperature and humidity controlled to mitigate evaporation of reagents. Additionally, it prevented contamination during amplicon extraction by self-containing the micromanipulation system. An image of the entire microwell array is also shown, as well as a micropipette probing a well. (d) Complex three-dimensional MDA amplicons were reduced to linear DNA using DNA polymerase I and Ampligase. This process substantially improved the complexity of the library during sequencing.
Figure 2
Depth of coverage of assembled contigs aligned to the reference E. coli genome. Three single E. coli cells were analyzed using MIDAS. Between 88% and 94% of the genome was assembled from 2–8M paired-end 100bp reads. Each colored circle is a histogram of the log2 of average depth of coverage across each assembled contig for one cell. Gaps are represented by blank whitespace in between colored contigs
Figure 3
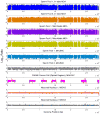
Genomic coverage of single bacterial (a,b) and mammalian (c,d) cells amplified by MDA in a tube and by MIDAS. The observed multi-peak profile for the MDA reactions implies that certain regions may have been amplified with exponentially greater bias compared to the majority of the genome. (a) Comparison of single E. coli cells amplified in a PCR tube for 10 hours (top), 2 hours (middle) and in a microwell (MIDAS) for 10 hours (bottom). Genomic positions were consolidated into 1 kb bins (x-axis), and were plotted against the log10 ratio (y-axis) of genomic coverage (normalized to the mean). (b) Distribution of coverage of amplified single bacterial cells. The x-axis shows the log10 ratio of genomic coverage normalized to the mean. (c) Comparison of single human cells amplified using traditional MDA in a PCR tube for 10 hours (top) or in a microwell (MIDAS) for 10 hours (middle) to a pool of unamplified human cells (bottom). Genomic positions were consolidated into variable bins of approximately 60 kb in size previously determined to contain a similar read count, and were plotted against the log10 ratio (y-axis) of genomic coverage (normalized to the mean). (d) Distribution of coverage of amplified single mammalian cells. The x-axis shows the log10 ratio of genomic coverage normalized to the mean.
Figure 4
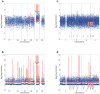
Detection of copy number variants using MIDAS (a,c) and in-tube MDA (b,d). Genomic positions were consolidated into bins of approximately 60 kb in size which were previously determined to contain a similar read count. Estimated copy numbers below were rounded to the nearest whole number. (a) Copy number variation in a Down Syndrome single cell analyzed with MIDAS. The x-axis shows genomic position, while the y-axis shows (on a log2 scale) the estimated copy number as a red line. The arrow indicates trisomy 21, which is clearly visible in this single cell. (b) Copy number variation in a Down Syndrome single cell analyzed with traditional in-tube MDA. The x-axis shows genomic position, while the y-axis shows (in a log2 scale) the estimated copy number as a red line. The arrow marks the expected region of Trisomy 21, which is not detectable in this data. (c) Copy number variation in a Down Syndrome single cell with Trisomy 21 “spike-ins.” The x-axis shows genomic position, while the y-axis shows (in a log2 scale) the estimated copy number as a red line. At each arrow, prior to CNV calling, data from a randomly determined 2 Mb section of Trisomy chromosome 21 was computationally inserted into the genome, simulating a small gain of single copy event. At each location, a copy number variant was called, showing that MIDAS can detect 2 Mb copy number variation accurately. (d) Copy number variation in a Down Syndrome single cell with Trisomy 21 “spike-ins.” The x-axis shows genomic position, while the y-axis shows (on a log2 scale) the estimated copy number as a red line. At each arrow, prior to CNV calling, data from a randomly determined 2 Mb section of Trisomy chromosome 21 was computationally inserted into the genome, simulating a small gain of single copy event.
Figure 5
Comparison of MIDAS to previously published data for in-tube MDA, microfluidic MDA and MALBAC.for diploid regions of pools of two sperm cells and diploid regions of a single SW480 cancer cell processed using MALBAC. Genomic positions were consolidated into variable bins of approximately 60 kb in size previously determined to contain a similar read count, and were plotted against the log10 ratio (y-axis) of genomic coverage (normalized to the mean). For the cancer cell data, non-diploid regions have been masked out (white gaps between pink) to remove the bias generated by comparing a highly aneuploid cell to a primarily diploid cell.
Similar articles
- Sequencing genomes from single cells by polymerase cloning.
Zhang K, Martiny AC, Reppas NB, Barry KW, Malek J, Chisholm SW, Church GM. Zhang K, et al. Nat Biotechnol. 2006 Jun;24(6):680-6. doi: 10.1038/nbt1214. Epub 2006 May 28. Nat Biotechnol. 2006. PMID: 16732271 - Single-cell genome sequencing at ultra-high-throughput with microfluidic droplet barcoding.
Lan F, Demaree B, Ahmed N, Abate AR. Lan F, et al. Nat Biotechnol. 2017 Jul;35(7):640-646. doi: 10.1038/nbt.3880. Epub 2017 May 29. Nat Biotechnol. 2017. PMID: 28553940 Free PMC article. - The properties and applications of single-molecule DNA sequencing.
Thompson JF, Milos PM. Thompson JF, et al. Genome Biol. 2011;12(2):217. doi: 10.1186/gb-2011-12-2-217. Epub 2011 Feb 24. Genome Biol. 2011. PMID: 21349208 Free PMC article. Review. - Mapping DNA polymerase errors by single-molecule sequencing.
Lee DF, Lu J, Chang S, Loparo JJ, Xie XS. Lee DF, et al. Nucleic Acids Res. 2016 Jul 27;44(13):e118. doi: 10.1093/nar/gkw436. Epub 2016 May 16. Nucleic Acids Res. 2016. PMID: 27185891 Free PMC article. - Massively parallel sequencing approaches for characterization of structural variation.
Koboldt DC, Larson DE, Chen K, Ding L, Wilson RK. Koboldt DC, et al. Methods Mol Biol. 2012;838:369-84. doi: 10.1007/978-1-61779-507-7_18. Methods Mol Biol. 2012. PMID: 22228022 Free PMC article. Review.
Cited by
- Comparison of Multiple Displacement Amplification (MDA) and Multiple Annealing and Looping-Based Amplification Cycles (MALBAC) in Limited DNA Sequencing Based on Tube and Droplet.
Zhou X, Xu Y, Zhu L, Su Z, Han X, Zhang Z, Huang Y, Liu Q. Zhou X, et al. Micromachines (Basel). 2020 Jun 29;11(7):645. doi: 10.3390/mi11070645. Micromachines (Basel). 2020. PMID: 32610698 Free PMC article. - A quantitative comparison of single-cell whole genome amplification methods.
de Bourcy CF, De Vlaminck I, Kanbar JN, Wang J, Gawad C, Quake SR. de Bourcy CF, et al. PLoS One. 2014 Aug 19;9(8):e105585. doi: 10.1371/journal.pone.0105585. eCollection 2014. PLoS One. 2014. PMID: 25136831 Free PMC article. - Singled out for sequencing.
Chi KR. Chi KR. Nat Methods. 2014 Jan;11(1):13-7. doi: 10.1038/nmeth.2768. Nat Methods. 2014. PMID: 24524130 No abstract available. - Improved multiple displacement amplification (iMDA) and ultraclean reagents.
Motley ST, Picuri JM, Crowder CD, Minich JJ, Hofstadler SA, Eshoo MW. Motley ST, et al. BMC Genomics. 2014 Jun 6;15(1):443. doi: 10.1186/1471-2164-15-443. BMC Genomics. 2014. PMID: 24906487 Free PMC article. - "Pop-slide" patterning: Rapid fabrication of microstructured PDMS gasket slides for biological applications.
Ramji R, Khan NT, Muñoz-Rojas A, Miller-Jensen K. Ramji R, et al. RSC Adv. 2015;5(81):66294-66300. doi: 10.1039/C5RA09056C. Epub 2015 Aug 4. RSC Adv. 2015. PMID: 26949529 Free PMC article.
References
- Zhang K, et al. Sequencing genomes from single cells by polymerase cloning. Nat Biotechnol. 2006;24:680–686. - PubMed
- Hou Y, et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell. 2012;148:873–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 HG005550/HG/NHGRI NIH HHS/United States
- R01 GM097253/GM/NIGMS NIH HHS/United States
- U01 MH098977/MH/NIMH NIH HHS/United States
- U01MH098977/MH/NIMH NIH HHS/United States
- R01GM097253/GM/NIGMS NIH HHS/United States
- R01HG004876/HG/NHGRI NIH HHS/United States
- T32 GM007752/GM/NIGMS NIH HHS/United States
- R01 HG004876/HG/NHGRI NIH HHS/United States
- P50HG005550/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous