Recurrent mutations, including NPM1c, activate a BRD4-dependent core transcriptional program in acute myeloid leukemia - PubMed (original) (raw)
doi: 10.1038/leu.2013.338. Epub 2013 Nov 13.
E J Gudgin 2, S J Horton 3, G Giotopoulos 3, E Meduri 3, S Robson 4, E Cannizzaro 5, H Osaki 3, M Wiese 5, S Putwain 3, C Y Fong 5, C Grove 6, J Craig 2, A Dittmann 7, D Lugo 8, P Jeffrey 8, G Drewes 7, K Lee 8, L Bullinger 9, R K Prinjha 8, T Kouzarides 4, G S Vassiliou 10, B J P Huntly 3
Affiliations
- PMID: 24220271
- PMCID: PMC3918873
- DOI: 10.1038/leu.2013.338
Free PMC article
Recurrent mutations, including NPM1c, activate a BRD4-dependent core transcriptional program in acute myeloid leukemia
M A Dawson et al. Leukemia. 2014 Feb.
Free PMC article
Abstract
Recent evidence suggests that inhibition of bromodomain and extra-terminal (BET) epigenetic readers may have clinical utility against acute myeloid leukemia (AML). Here we validate this hypothesis, demonstrating the efficacy of the BET inhibitor I-BET151 across a variety of AML subtypes driven by disparate mutations. We demonstrate that a common 'core' transcriptional program, which is HOX gene independent, is downregulated in AML and underlies sensitivity to I-BET treatment. This program is enriched for genes that contain 'super-enhancers', recently described regulatory elements postulated to control key oncogenic driver genes. Moreover, our program can independently classify AML patients into distinct cytogenetic and molecular subgroups, suggesting that it contains biomarkers of sensitivity and response. We focus AML with mutations of the Nucleophosmin gene (NPM1) and show evidence to suggest that wild-type NPM1 has an inhibitory influence on BRD4 that is relieved upon NPM1c mutation and cytosplasmic dislocation. This leads to the upregulation of the core transcriptional program facilitating leukemia development. This program is abrogated by I-BET therapy and by nuclear restoration of NPM1. Finally, we demonstrate the efficacy of I-BET151 in a unique murine model and in primary patient samples of NPM1c AML. Taken together, our data support the use of BET inhibitors in clinical trials in AML.
Figures
Figure 1
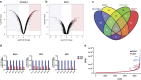
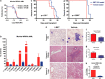
I-BET151 has activity in a broad range of AML (a) A panel of human AML cell lines encompassing a variety of oncogenic drivers were tested in cell proliferation assays using I-BET151. We have previously reported some of this data and report it again only to provide an overall appreciation of sensitivity of AML cell lines to I-BET151. (b) Clonogenic assays performed in cytokine-supplemented methylcellulose in the presence of vehicle (dimethyl sulfoxide (DMSO)) or I-BET151 show a marked reduction in colony numbers (enumerated in the bar graph) after treatment with I-BET151. (c) Primary murine hematopoietic progenitors were isolated from mouse bone marrow and retrovirally transformed with MOZ-TIF2 or NUP98-HOXA9. These cells were propagated in liquid culture as well as being used in clonogenic assays. Both proliferation and clonogenic assays (enumerated in the bar graph) demonstrate a marked sensitivity to I-BET151. (d) The degree of apoptosis in OCI-AML3 was assessed using the vital dye 7-amino-actinomycin D (7-AAD) and Annexin V in cells following 72 h incubation with DMSO or I-BET. These data demonstrate a marked induction of apoptosis. (e) Cell cycle progression in OCI-AML3 was assessed 24 h after incubation with DMSO or I-BET151. These data demonstrate a marked increase in G0/G1 fraction, which was accompanied by a concomitant decrease in the number of cells in S and G2/M phases. (f) Clonogenic assays with primary human AML cells from five different patients (Supplementary Table S2). Cells were plated in cytokine-supplemented methylcellulose in the presence of vehicle (DMSO) or I-BET151. These show a marked reduction of colony formation in the presence of I-BET151. AML patient samples demonstrate apoptosis following treatment with I-BET. A representative sample is shown (g) and the results from five separate patients are enumerated in the bar graph (h).
Figure 2
A core transcriptional program is affected by I-BET151 in AML. (a) OCI-AML3 and (b) SKM1 cells were treated for six hours with either I-BET151 or DMSO (vehicle) followed by mRNA extraction. The mRNA from three biological replicates was used to generate gene expression data set. Volcano plots for the DMSO- versus I-BET151-treated samples, showing the adjusted significance _P_-value (log10) versus the fold change (log2) are shown. These plots identify a small subset of genes that demonstrate a significant change in expression (_P_⩽0.01). This is represented as either twofold downregulation (blue) or twofold upregulation (red) on treatment with I-BET151. (c) Venn diagram of all the significantly downregulated genes, shows that 26 genes are commonly downregulated in all four cell lines. Several of these genes are also downregulated in another sensitive AML cell lines KG-1 (Supplementary Figure S3). (d) Similar transcriptional changes were demonstrated in both NPM1c mutated and wild-type AML patients, for exemplar, genes C-MYC, BCL2 and IRF8. (e) Total BRD4 ChIP-seq signal in units of reads per million is charted at all enhancer regions. Enhancers are ranked by increasing BRD4 ChIP-seq signal in the presence (red) or absence of I-BET151 (blue). Super-enhancers are enriched in the vertically rising ranked enhancers to the right of the graph. Treatment with I-BET151 markedly decreases the BRD4 read count at these enhancers.
Figure 3
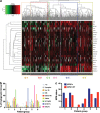
The core transcriptional program classifies human AML: (a) 18 of 26 genes (80%) from the BET-responsive core signature were differentially expressed across a cohort of 436 AML patients as shown in the heat map. The gene set could classify this cohort into six groups through the use of unsupervised clustering. (b) Significant differences in cytogenetic characteristics were shown for individuals in each of the groups (P<0.0001), with significant differences in molecular prognostic factors including mutational status for (c) NPM1c and FLT3-ITD (Supplementary Figure 3) also noted (_P_=0.02 and _P_=0.02, respectively). (+8=trisomy 8, NK=normal karyotype, Other HR=other high risk (t(6;9), 3q abnormality and del 5q).
Figure 4
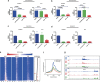
Nuclear relocalization of NPM1c phenocopies treatment with I-BET151: treatment with LMB reduces the expression of (a) BCL2 and (b) MYC in OCI-AML3 but not KG-1. In contrast, I-BET151 reduces the expression of these genes in both cell lines. The gene expression changes shown were performed by real-time PCR (RT-PCR) on cDNA prepared from independent biological replicates. The expression level of target genes in the presence of DMSO was assigned a value of 100 following normalization to the B2-microglobulin (B2M) house-keeping gene whose expression in all cell lines is unaltered by I-BET151 or LMB treatment. The fold-change following treatment with I-BET151 or LMB for 6 h is shown (after normalization to the B2M house-keeping gene). Chromatin prepared from OCI-AML3 cells after 6 h of treatment with DMSO, LMB or I-BET151 was used in chromatin immunoprecipitation (ChIP) assays, followed by real-time PCR analysis. In comparison with DMSO, LMB reduces the chromatin binding of BRD4 at the transcriptional start site (TSS) of (c) BCL2 and (d) MYC in OCI-AML3 but not KG-1. In contrast, I-BET151 reduces BRD4 binding at both these target genes in both cell lines. Bar graphs are represented as the mean enrichment relative to input and error bars reflect s.d. of results derived from biological triplicate experiments. (e) Density of BRD4 ChIP-seq reads in OCI-AML3 shown as heat maps centered on the TSS of annotated genes with 5 kb of flanking sequence either side. Heat maps are shown for BRD4 binding following treatment with DMSO, I-BET151 and LMB. Red color indicates higher density of reads. The decrease in BRD4 binding occurs primarily over genes that show a significant decrease in expression following treatment with I-BET151 (red dotted line). (f) Mean enrichment pattern for BRD4 binding was profiled across all annotated TSSs following treatment of OCI-AML3 with DMSO, I-BET151 and LMB. These data demonstrate that similar to treatment with I-BET151, the relocation of NPM1c with LMB reduces BRD4 binding at chromatin. (g) The decrease in BRD4 binding by LMB and I-BET151 is demonstrated across the BCL2 and MYC loci.
Figure 5
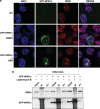
Relocation of NPM1c into the nucleus leads to a re-association with BRD4: in a cell line diploid for wild-type NPM1 we trasfected mutant NPM1 N-terminally tagged with green fluorescent protein (GFP-NPM1c). Transfection efficiency was between 10–20%. We were able to distinguish wild-type NPM1 from mutant NPM1 with an antibody raised against amino acids 1–100 of NPM1 and therefore does not recognize GFP-NPM1c. In this isogenic cellular background (a) confocal immunofluorescence microscopy images show that the subcellular localization of NPM1c is within the cytoplasm. However, following treatment with LMB, NPM1c is relocated back into the nucleus/nucleolus. (b) From the subset (10–20%) of cells expressing GFP-NPM1c, we demonstrate that the relocalization of NPM1c into the nucleus/nucleolus leads to an increased association with BRD4. Also demonstrated is 5% input and 5% of the flow through (FT) fraction following immunoprecipitaion (IP).
Figure 6
I-BET151 is efficacious in vitro and in vivo in a murine model of NPM1c AML and primary human NPM1c AML samples. Six different murine NPM1c AML were tested in (a) cell proliferation and (b) clonogenic assays. These data demonstrate that I-BET151 is effective in vitro in multiple NPM1c AML cases that carry a variety of other collaborating mutations. (c) Kaplan–Meier curve demonstrating that treatment of NOD-SCID mice transplanted with 1 × 107 murine NPM1c leukemic cells show a significant increase in overall survival following treatment with I-BET151 at the experimental end point. Here, 24 mice were split into three equal groups and transplanted with three different NPM1c AML. Half of each group were treated with vehicle and half treated with I-BET151. Treatment was commenced on day 10 post transplantation. (d) Kaplan–Meier curve from the subgroup of mice that received the NPM1c AML, which contained a concurrent gain of function mutation in FLT3. These data show a significant increase in overall survival following treatment with I-BET151 at the experimental end point. (e) Top panel—Romanowsky stain of a peripheral blood smear from a vehicle- and I-BET151-treated mouse showing the morphological appearance of the increased circulating leukemic cells in the control mice. Middle panel—Haematoxylin and eosin stained histological sections of the renal parenchyma and lung (lower panel) of control and treated mice. These data demonstrate overt extramedullary leukemic infiltration of the kidney and lung in the control mouse. In contrast, a relatively normal architecture is seen in the treated animal. (f) Spleen weights and (g) total circulating white cell count (WCC) from all the vehicle and treated mice at the time of necropsy. (h) Clonogenic assays with 5 × 103–1 × 104 primary human NPM1c AML cells from five different patients. Cells were plated in cytokine-supplemented methylcellulose in the presence of vehicle (DMSO) or I-BET151. These show a marked reduction of colony formation in the presence of I-BET151.
Figure 7
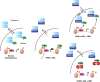
Model for the molecular mechanism of action for I-BET in NPM1c AML. Wild-type nucleophosmin 1 (NPM1) associates with a small nuclear pool of BRD4 (left panel) and exerts an inhibitory effect on its transcriptional activity. The NPM1c mutation in AML alters this equilibrium (middle panel) as a significant proportion of NPM1 is dislocated into the cytoplasm without BRD4, which is then free to drive the transcription of its target genes. I-BET displaces the binding of BRD4 from chromatin (right upper panel) leading to the repression of the target genes and relocation of NPM1c into the nucleus with LMB phenocopies I-BET (left lower panel), as it leads to a re-association of NPM1c with BRD4 off the chromatin template also resulting in transcriptional repression.
Similar articles
- Therapeutic targeting in pediatric acute myeloid leukemia with aberrant HOX/MEIS1 expression.
Juul-Dam KL, Shukla NN, Cooper TM, Cuglievan B, Heidenreich O, Kolb EA, Rasouli M, Hasle H, Zwaan CM. Juul-Dam KL, et al. Eur J Med Genet. 2023 Dec;66(12):104869. doi: 10.1016/j.ejmg.2023.104869. Epub 2023 Oct 29. Eur J Med Genet. 2023. PMID: 38174649 Free PMC article. Review. - Imidazoquinoxaline derivative EAPB0503: A promising drug targeting mutant nucleophosmin 1 in acute myeloid leukemia.
Nabbouh AI, Hleihel RS, Saliba JL, Karam MM, Hamie MH, Wu HJM, Berthier CP, Tawil NM, Bonnet PA, Deleuze-Masquefa C, El Hajj HA. Nabbouh AI, et al. Cancer. 2017 May 1;123(9):1662-1673. doi: 10.1002/cncr.30515. Epub 2017 Jan 5. Cancer. 2017. PMID: 28055106 - Highly active combination of BRD4 antagonist and histone deacetylase inhibitor against human acute myelogenous leukemia cells.
Fiskus W, Sharma S, Qi J, Valenta JA, Schaub LJ, Shah B, Peth K, Portier BP, Rodriguez M, Devaraj SG, Zhan M, Sheng J, Iyer SP, Bradner JE, Bhalla KN. Fiskus W, et al. Mol Cancer Ther. 2014 May;13(5):1142-54. doi: 10.1158/1535-7163.MCT-13-0770. Epub 2014 Jan 16. Mol Cancer Ther. 2014. PMID: 24435446 - Mutant NPM1 Maintains the Leukemic State through HOX Expression.
Brunetti L, Gundry MC, Sorcini D, Guzman AG, Huang YH, Ramabadran R, Gionfriddo I, Mezzasoma F, Milano F, Nabet B, Buckley DL, Kornblau SM, Lin CY, Sportoletti P, Martelli MP, Falini B, Goodell MA. Brunetti L, et al. Cancer Cell. 2018 Sep 10;34(3):499-512.e9. doi: 10.1016/j.ccell.2018.08.005. Cancer Cell. 2018. PMID: 30205049 Free PMC article. - Perspectives for therapeutic targeting of gene mutations in acute myeloid leukaemia with normal cytogenetics.
Falini B, Sportoletti P, Brunetti L, Martelli MP. Falini B, et al. Br J Haematol. 2015 Aug;170(3):305-22. doi: 10.1111/bjh.13409. Epub 2015 Apr 19. Br J Haematol. 2015. PMID: 25891481 Review.
Cited by
- Therapeutic targeting in pediatric acute myeloid leukemia with aberrant HOX/MEIS1 expression.
Juul-Dam KL, Shukla NN, Cooper TM, Cuglievan B, Heidenreich O, Kolb EA, Rasouli M, Hasle H, Zwaan CM. Juul-Dam KL, et al. Eur J Med Genet. 2023 Dec;66(12):104869. doi: 10.1016/j.ejmg.2023.104869. Epub 2023 Oct 29. Eur J Med Genet. 2023. PMID: 38174649 Free PMC article. Review. - Pharmacological Targeting of BET Bromodomain Proteins in Acute Myeloid Leukemia and Malignant Lymphomas: From Molecular Characterization to Clinical Applications.
Reyes-Garau D, Ribeiro ML, Roué G. Reyes-Garau D, et al. Cancers (Basel). 2019 Oct 2;11(10):1483. doi: 10.3390/cancers11101483. Cancers (Basel). 2019. PMID: 31581671 Free PMC article. Review. - Transcriptional targeting of oncogene addiction in medullary thyroid cancer.
Valenciaga A, Saji M, Yu L, Zhang X, Bumrah C, Yilmaz AS, Knippler CM, Miles W, Giordano TJ, Cote GJ, Ringel MD. Valenciaga A, et al. JCI Insight. 2018 Aug 23;3(16):e122225. doi: 10.1172/jci.insight.122225. eCollection 2018 Aug 23. JCI Insight. 2018. PMID: 30135308 Free PMC article. - Dual-target inhibitors of bromodomain and extra-terminal proteins in cancer: A review from medicinal chemistry perspectives.
Feng L, Wang G, Chen Y, He G, Liu B, Liu J, Chiang CM, Ouyang L. Feng L, et al. Med Res Rev. 2022 Mar;42(2):710-743. doi: 10.1002/med.21859. Epub 2021 Oct 11. Med Res Rev. 2022. PMID: 34633088 Free PMC article. Review. - BRD4 bimodal binding at promoters and drug-induced displacement at Pol II pause sites associates with I-BET sensitivity.
Khoueiry P, Ward Gahlawat A, Petretich M, Michon AM, Simola D, Lam E, Furlong EE, Benes V, Dawson MA, Prinjha RK, Drewes G, Grandi P. Khoueiry P, et al. Epigenetics Chromatin. 2019 Jul 2;12(1):39. doi: 10.1186/s13072-019-0286-5. Epigenetics Chromatin. 2019. PMID: 31266503 Free PMC article.
References
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. - PubMed
- Rosenbauer F, Tenen DG. Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol. 2007;7:105–117. - PubMed
- Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. N Engl J Med. 2012;367:647–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 079249/WT_/Wellcome Trust/United Kingdom
- 092096/WT_/Wellcome Trust/United Kingdom
- 10827/CRUK_/Cancer Research UK/United Kingdom
- 095663/WT_/Wellcome Trust/United Kingdom
- 100140/WT_/Wellcome Trust/United Kingdom
- 17001/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical