Prolonged bioluminescence monitoring in mouse ex vivo bone culture revealed persistent circadian rhythms in articular cartilages and growth plates - PubMed (original) (raw)
Prolonged bioluminescence monitoring in mouse ex vivo bone culture revealed persistent circadian rhythms in articular cartilages and growth plates
Naoki Okubo et al. PLoS One. 2013.
Abstract
The bone is a metabolically active organ which undergoes repeated remodeling cycles of bone resorption and formation. In this study, we revealed a robust and extremely long-lasting circadian rhythm in ex vivo culture maintained for over six months from the femoral bone of a PERIOD2(Luciferase) mouse. Furthermore, we also identified robust circadian clocks in flat bones. High- or low-magnification real-time bioluminescence microscopic imaging revealed that the robust circadian rhythms emanated from the articular cartilage and the epiphyseal cartilage within the growth plate of juvenile animals. Stimulation by forskolin or dexamethasone treatment caused type 0 phase resetting, indicating canonical entraining properties of the bone clock. Together, our findings from long-term ex vivo culture revealed that "tissue-autonomous" circadian rhythm in the articular cartilage and the growth plate of femoral bone functions for several months even in an organ culture condition, and provided a useful in vitro assay system investigating the role of the biological clock in bone formation or development.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
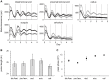
Figure 1. Persistent cell-autonomous oscillators in the bone.
(A) Mouse femurs harvested from PER2Luc mice. Distal femoral ends were used. (B) Two representative (black and grey dots) bioluminescence circadian rhythm traces. (C–D) Medium change restored circadian oscillation after 280 days in ex vivo culture. Four representative periods are shown where data are expressed as a percent of the max value in 7 days (C), and the entire 292 days of ex vivo culture data are shown in D. Data were detrended by a 24-hour moving average and the grey area is equivalent to data shown in C. Arrowheads indicate medium change.
Figure 2. Circadian clocks in different bone tissues.
(A) Bioluminescence circadian rhythms obtained from the distal femoral end, proximal femoral end, radius, scapula, and calvarium. Color dots (light gray, gray and black) indicate traces from three independent samples. (B–C) The quantitative analysis of circadian bioluminescence rhythms. The period length (B) and bioluminescence peak phase (C) are shown (mean ± SEM, n = 3). For peak phase calculation, CT0 was set as a start time of the measurement. CT: Circadian time; dis fem: distal femoral end; pro fem: proximal femoral end; rad: radius; sca: scapula; cal: calvarium.
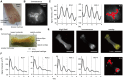
Figure 3. Microscopic observation of the femur.
(A) The overview of the distal half of the femur. The dotted line indicates epiphyseal cartilage. The mirror-reversed image was used for comparison to the bioluminescence image. A sample was obtained from a 16.5-week old mouse and cultured for 6 days before observation. (B) The bioluminescence image obtained by a microscope-based high sensitivity CCD camera system. (C) A time series analysis of the epiphyseal cartilage (ROI-1) and femoral trochlea (ROI-2). The right panel shows set ROIs. (D) A schematic view of the femur. The dotted line indicates epiphyseal cartilage. A sample was obtained from a 2.4-week old mouse and cultured for 4 days before observation. (E) A representative microscopic view of the femur. A bright field (left), bioluminescence (middle), and overlaid image (right) are displayed. For the overlaid image, the bioluminescence signal is shown in yellow. (F) A time series analysis of the signal intensity for 88-hours. The right panel shows set ROIs.
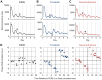
Figure 4. Phase resetting of bone circadian clocks by chemicals.
(A–C) Effects of forskolin and dexamethasone (DEX) on circadian clocks. Representative data showing the phase advancement (upper panels) or phase delay of the bone circadian clock (lower panels). The arrowhead indicates chemical administration. From left to right, the vehicle (ethanol; EtOH) (A), DEX (B), or forskolin (C). (D) Phase response curves for the vehicle (left), forskolin (middle) or DEX (right). Results of 20 (vehicle), 27 (forskolin), and 30 (Dex) experiments were plotted, respectively. The horizontal axis represents time relative to PER2::Luc peak time (CT12) and the vertical axis represents phase shift. Data are represented in circadian hours (1 circadian hour = period length (hour) /24).
Similar articles
- A PTH-responsive circadian clock operates in ex vivo mouse femur fracture healing site.
Kunimoto T, Okubo N, Minami Y, Fujiwara H, Hosokawa T, Asada M, Oda R, Kubo T, Yagita K. Kunimoto T, et al. Sci Rep. 2016 Feb 29;6:22409. doi: 10.1038/srep22409. Sci Rep. 2016. PMID: 26926165 Free PMC article. - Parathyroid hormone resets the cartilage circadian clock of the organ-cultured murine femur.
Okubo N, Fujiwara H, Minami Y, Kunimoto T, Hosokawa T, Umemura Y, Inokawa H, Asada M, Oda R, Kubo T, Yagita K. Okubo N, et al. Acta Orthop. 2015;86(5):627-31. doi: 10.3109/17453674.2015.1029393. Acta Orthop. 2015. PMID: 25765847 Free PMC article. - Real-Time Monitoring of Circadian Rhythms in the Eye.
Baba K, Tosini G. Baba K, et al. Methods Mol Biol. 2022;2550:367-375. doi: 10.1007/978-1-0716-2593-4_37. Methods Mol Biol. 2022. PMID: 36180706 - Monitoring cell-autonomous circadian clock rhythms of gene expression using luciferase bioluminescence reporters.
Ramanathan C, Khan SK, Kathale ND, Xu H, Liu AC. Ramanathan C, et al. J Vis Exp. 2012 Sep 27;(67):4234. doi: 10.3791/4234. J Vis Exp. 2012. PMID: 23052244 Free PMC article. - Circadian Clocks in Articular Cartilage and Bone: A Compass in the Sea of Matrices.
Yang N, Meng QJ. Yang N, et al. J Biol Rhythms. 2016 Oct;31(5):415-27. doi: 10.1177/0748730416662748. Epub 2016 Aug 23. J Biol Rhythms. 2016. PMID: 27558096 Review.
Cited by
- Regulation and Role of Transcription Factors in Osteogenesis.
Chan WCW, Tan Z, To MKT, Chan D. Chan WCW, et al. Int J Mol Sci. 2021 May 21;22(11):5445. doi: 10.3390/ijms22115445. Int J Mol Sci. 2021. PMID: 34064134 Free PMC article. Review. - Hypophosphatemia Regulates Molecular Mechanisms of Circadian Rhythm.
Noguchi T, Hussein AI, Horowitz N, Carroll D, Gower AC, Demissie S, Gerstenfeld LC. Noguchi T, et al. Sci Rep. 2018 Sep 13;8(1):13756. doi: 10.1038/s41598-018-31830-7. Sci Rep. 2018. PMID: 30213970 Free PMC article. - Sleep Disruption and Bone Health.
Swanson C. Swanson C. Curr Osteoporos Rep. 2022 Jun;20(3):202-212. doi: 10.1007/s11914-022-00733-y. Epub 2022 Apr 30. Curr Osteoporos Rep. 2022. PMID: 35488985 Free PMC article. Review. - Robust Circadian Rhythm and Parathyroid Hormone-Induced Resetting during Hypertrophic Differentiation in ATDC5 Chondroprogenitor Cells.
Hosokawa T, Tsuchiya Y, Okubo N, Kunimoto T, Minami Y, Fujiwara H, Umemura Y, Koike N, Kubo T, Yagita K. Hosokawa T, et al. Acta Histochem Cytochem. 2015 Dec 25;48(6):165-71. doi: 10.1267/ahc.15025. Epub 2015 Nov 10. Acta Histochem Cytochem. 2015. PMID: 26855448 Free PMC article.
References
- Nih Consensus Development Panel on Osteoporosis Prevention D, Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785–795. - PubMed
- Swaminathan R (2001) Biochemical markers of bone turnover. Clin Chim Acta 313: 95–105. - PubMed
- Vergely N, Lafage-Proust MH, Caillot-Augusseau A, Millot L, Lang F, et al. (2002) Hypercorticism blunts circadian variations of osteocalcin regardless of nutritional status. Bone 30: 428–435. - PubMed
- Ju HS, Leung S, Brown B, Stringer MA, Leigh S, et al. (1997) Comparison of analytical performance and biological variability of three bone resorption assays. Clin Chem 43: 1570–1576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases