Smac mimetics induce inflammation and necrotic tumour cell death by modulating macrophage activity - PubMed (original) (raw)
Smac mimetics induce inflammation and necrotic tumour cell death by modulating macrophage activity
D Lecis et al. Cell Death Dis. 2013.
Abstract
Smac mimetics (SMs) comprise a class of small molecules that target members of the inhibitor of apoptosis family of pro-survival proteins, whose expression in cancer cells hinders the action of conventional chemotherapeutics. Herein, we describe the activity of SM83, a newly synthesised dimeric SM, in two cancer ascites models: athymic nude mice injected intraperitoneally with IGROV-1 human ovarian carcinoma cells and immunocompetent BALB/c mice injected with murine Meth A sarcoma cells. SM83 rapidly killed ascitic IGROV-1 and Meth A cells in vivo (prolonging mouse survival), but was ineffective against the same cells in vitro. IGROV-1 cells in nude mice were killed within the ascites by a non-apoptotic, tumour necrosis factor (TNF)-dependent mechanism. SM83 administration triggered a rapid inflammatory event characterised by host secretion of TNF, interleukin-1β and interferon-γ. This inflammatory response was associated with the reversion of the phenotype of tumour-associated macrophages from a pro-tumoural M2- to a pro-inflammatory M1-like state. SM83 treatment was also associated with a massive recruitment of neutrophils that, however, was not essential for the antitumoural activity of this compound. In BALB/c mice bearing Meth A ascites, SM83 treatment was in some cases curative, and these mice became resistant to a second injection of cancer cells, suggesting that they had developed an adaptive immune response. Altogether, these results indicate that, in vivo, SM83 modulates the immune system within the tumour microenvironment and, through its pro-inflammatory action, leads cancer cells to die by necrosis with the release of high-mobility group box-1. In conclusion, our work provides evidence that SMs could be more therapeutically active than expected by stimulating the immune system.
Figures
Figure 1
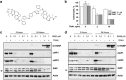
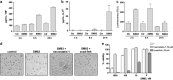
SM83 induces apoptosis in vitro when combined with TRAIL. (a) Chemical structure of the dimeric SM SM83. (b) IGROV-1 cells were treated with 0.1 or 1.0 _μ_M SM83 alone or in combination with 2 or 10 ng/ml TRAIL. Cell growth is expressed as a percentage relative to cells mock-treated with vehicle. Values are mean and S.D. from one experiment representative experiment of three performed. (c and d) Western blot analysis of XIAP, cIAP1, cIAP2 and cleaved PARP (Cl PARP) in IGROV-1 cells treated 3 or 24 h with SM83 (c) or SM59 (SM-164) (d) in the absence or presence of 10 ng/ml TRAIL. Actin is shown as loading control. Arrow, specific XIAP band
Figure 2
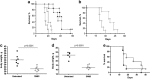
Treatment with SM83 in monotherapy increases the survival of mice bearing cancer ascites. (a) Nude mice were injected i.p. with IGROV-1 cells and left untreated (○) or treated 5 times a week, for 2 consecutive weeks starting the day after injection, with 5 mg/kg SM83 (▴), 2.5 mg/kg TRAIL (∇) or with the same doses of SM83 and TRAIL together (▪). One experiment representative of two performed is shown. Each treatment group contained seven mice. Survival curve for SM83-treated mice and controls. (b) Survival curve for SM59-treated and control mice. Untreated (○) or treated with SM SM59 (∇). (c) The formation of ascites was checked by monitoring body weight on day 17. (d and e) BALB/c mice were injected with Meth A cells and, starting on day 7, were treated daily with 5 mg/kg SM83. (d) The formation of ascites was checked by monitoring body weight on day 13. The horizontal line represents the mean. (e) Survival curve for untreated (•) or SM83-treated mice (○) starting from day 7
Figure 3
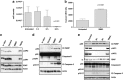
SM83 kills ascites tumour cells through a rapid, non-apoptotic event. Ascitic fluids were collected from nude mice injected with IGROV-1 cells and left untreated or treated with a single injection of SM83 (5 mg/kg). (a) Number of tumour cells within the ascites of untreated animals (_n_=11) or of animals treated for 3 (_n_=4), 6 (_n_=4) and 24 h (_n_=9). Data are mean and S.D. (b) Levels of human cytokeratin-18 in the serum collected 24 h after a single dose of SM83 (5 mg/kg) or no treatment. (c) Western blot of apoptosis markers in IGROV-1 cells cultured in vitro or recovered from the ascites of control mice (_n_=2) or mice treated with SM83 (_n_=2) or TRAIL for 24 h. (d) Western blot to detect activated apoptosis markers cleaved PARP (p89), caspase-8 (precursor p55 and cleaved form p43/41) and cleaved caspase-3 (p19/p17) in IGROV-1 cells cultured in vitro or recovered from the ascites (24 h) of control mice (_n_=2) and of mice treated with 5 mg/kg SM83 (_n_=2) or 10 mg/kg TRAIL. (e) Western blot of apoptotic markers in tumour cells collected from the ascites of untreated mice (_n_=2) or from mice treated for 3 or 6 h with SM83 (_n_=2 for both) or with TRAIL for 24 h. Arrow, specific band for XIAP
Figure 4
SM83 treatment induces the expression of inflammatory cytokines in vivo. Nude mice were injected i.p. with IGROV-1 cells and treated or not with a single dose of 5 mg/kg SM83; ascites was collected 3, 6 and 24 h after SM83 administration. (a) SM83 transiently increased the level of murine TNF measured by ELISA. (b) SM83 treatment reduced ascites cell counts but this action was blocked when TNF was sequestered with the higher dose of etanercept (P_=0.0118 relative to treated with SM83 alone). (c) Western blots of ascites tumour cell protein from untreated mice and mice treated with SM83 alone or in combination with 150 mg/kg etanercept. Arrow, specific band for XIAP. (d–h) ELISA results for IL-1_β (d), IFN-γ (e), IL-10 (f), transforming growth factor-β (TGF-β) (g) and IL-4 (h) in the ascitic fluid of mice treated as above. Results for IL-10 are shown as fold induction owing to the lack of recombinant protein standard
Figure 5
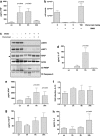
SM treatment activates macrophage and sensitises them to necroptotic death. (a and b) BALB/c BMDM (5 × 105) were seeded in 96-well plates, left to adhere for 16 h and treated or not with 1 μ_M SM83 for up to 24 h. SM83 treatment promoted the secretion of the pro-inflammatory cytokines TNF (a) and IL-1_β (b). (c) NF-_κ_B activation by SM83 evaluated in the macrophage cell line RAW stably transfected with a NF-_κ_B luciferase reporter gene. One representative experiment of two performed is shown. Values are mean and S.D.; _n_=3. (d and e) BALB/c BMDM were seeded in six-well plates, left to adhere for 16 h and treated with serial dilutions of SM83 in the absence or presence of necrostatin-1 or z-vad-fmk to inhibit necroptosis or apoptosis, respectively. (d) Representative images showing cell morphology after 24 h of treatment. (e) Cell viability after treatments evaluated using the CellTiter-Glo assay
Figure 6
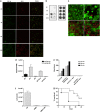
SM83 treatment promotes peritoneal neutrophil recruitment and activation. Cells were harvested from the ascitic fluid of mice treated with 5 mg/kg SM83. (a) Immunofluorescence for CD11b (green) and Gr-1 (red) was performed on Cytospin cell preparations. SM83 treatment induced a massive recruitment of neutrophils (CD11b+ Gr-1+) at 24 h but not at earlier time points. (b) Dot blot of HMGB-1 (left panel) was performed on cleared ascitic fluids collected from untreated (un) or treated mice at 3, 6 and 24 h after a single injection of 5 mg/kg SM83. Right panel, loading control. (c) Infiltration of neutrophils (Gr-1; red) and HMGB-1 expression in dying tumour cells (green) in ascites untreated (upper row) and treated for 24 h (lower row) with a single injection of SM83. Left and right panels show two different magnifications ( × 10 and × 40). (d) PMN migration assessed by Transwell assay. Wild-type PMN from BALB/c mice (WT) or TNF-R1-deficient PMN from TNF-R1-KO mice (KO) were seeded into the upper chamber in the absence or presence of the HMGB-1 inhibitor glycyrrhizin (GLZ), whereas the ascitic fluid of mice untreated or treated for 6 h (1:20 in medium) was added to the lower chamber of the Transwell insert. PMN were left to migrate overnight, harvested and counted. (e) Activation of wild-type PMNs was colorimetrically evaluated on the basis of the ability of cells to oxidise the cytochrome c in presence of the ascitic fluid collected from mice 6 h after treating with a single injection of SM83. The experiment was also performed in the presence of GLZ and the TNF inhibitor etanercept. (f) Tumour cell counts in the ascites of untreated mice (UN), mice treated with a single injection of SM83 alone or mice treated with SM83 24 h after depletion of neutrophils by injection with the 1A8 mAb. (g) BALB/c mice were injected i.p. with Meth A cells and treated with 1A8 alone (▪) or in combination with 5 mg/kg SM83 (□), starting from day 7
Figure 7
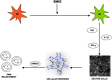
Proposed mechanism of action of SM83 in cancer ascites. SM83 stimulates the reversal of macrophages from M2 to M1 phenotype. TNF secreted by M1 macrophages triggers necrotic death of the cancer cells within the ascitic fluid; the dying cells release HMGB-1 that, together with TNF, recruits neutrophils
Similar articles
- Antitumor activity of a novel homodimeric SMAC mimetic in ovarian carcinoma.
Gatti L, De Cesare M, Ciusani E, Corna E, Arrighetti N, Cominetti D, Belvisi L, Potenza D, Moroni E, Vasile F, Lecis D, Delia D, Castiglioni V, Scanziani E, Seneci P, Zaffaroni N, Perego P. Gatti L, et al. Mol Pharm. 2014 Jan 6;11(1):283-93. doi: 10.1021/mp4004578. Epub 2013 Nov 27. Mol Pharm. 2014. PMID: 24256025 - Anti-inflammatory and antitumoural effects of Uncaria guianensis bark.
Urdanibia I, Michelangeli F, Ruiz MC, Milano B, Taylor P. Urdanibia I, et al. J Ethnopharmacol. 2013 Dec 12;150(3):1154-62. doi: 10.1016/j.jep.2013.10.055. Epub 2013 Nov 7. J Ethnopharmacol. 2013. PMID: 24212077 - USP11-dependent selective cIAP2 deubiquitylation and stabilization determine sensitivity to Smac mimetics.
Lee EW, Seong D, Seo J, Jeong M, Lee HK, Song J. Lee EW, et al. Cell Death Differ. 2015 Sep;22(9):1463-76. doi: 10.1038/cdd.2014.234. Epub 2015 Jan 23. Cell Death Differ. 2015. PMID: 25613375 Free PMC article. - Smac mimetics as new cancer therapeutics.
Chen DJ, Huerta S. Chen DJ, et al. Anticancer Drugs. 2009 Sep;20(8):646-58. doi: 10.1097/CAD.0b013e32832ced78. Anticancer Drugs. 2009. PMID: 19550293 Review. - Smac mimetics as IAP antagonists.
Fulda S. Fulda S. Semin Cell Dev Biol. 2015 Mar;39:132-8. doi: 10.1016/j.semcdb.2014.12.005. Epub 2014 Dec 27. Semin Cell Dev Biol. 2015. PMID: 25550219 Review.
Cited by
- Therapeutics Targeting the Core Apoptotic Machinery.
Hamilton C, Fox JP, Longley DB, Higgins CA. Hamilton C, et al. Cancers (Basel). 2021 May 26;13(11):2618. doi: 10.3390/cancers13112618. Cancers (Basel). 2021. PMID: 34073507 Free PMC article. Review. - Mitochondrial control of inflammation.
Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Marchi S, et al. Nat Rev Immunol. 2023 Mar;23(3):159-173. doi: 10.1038/s41577-022-00760-x. Epub 2022 Jul 25. Nat Rev Immunol. 2023. PMID: 35879417 Free PMC article. Review. - TRAF3: a novel tumor suppressor gene in macrophages.
Lalani AI, Luo C, Han Y, Xie P. Lalani AI, et al. Macrophage (Houst). 2015 Sep 30;2:e1009. doi: 10.14800/macrophage.1009. Macrophage (Houst). 2015. PMID: 26661944 Free PMC article. - The inhibitor of apoptosis proteins antagonist Debio 1143 promotes the PD-1 blockade-mediated HIV load reduction in blood and tissues of humanized mice.
Bobardt M, Kuo J, Chatterji U, Wiedemann N, Vuagniaux G, Gallay P. Bobardt M, et al. PLoS One. 2020 Jan 24;15(1):e0227715. doi: 10.1371/journal.pone.0227715. eCollection 2020. PLoS One. 2020. PMID: 31978106 Free PMC article. - Loss of cIAP1 in Endothelial Cells Limits Metastatic Extravasation through Tumor-Derived Lymphotoxin Alpha.
Vasilikos L, Hänggi K, Spilgies LM, Kisele S, Rufli S, Wong WW. Vasilikos L, et al. Cancers (Basel). 2021 Feb 3;13(4):599. doi: 10.3390/cancers13040599. Cancers (Basel). 2021. PMID: 33546280 Free PMC article.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
- Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. - PubMed
- Lopez J, John SW, Tenev T, Rautureau GJ, Hinds MG, Francalanci F, et al. CARD-mediated autoinhibition of cIAP1's E3 ligase activity suppresses cell proliferation and migration. Mol Cell. 2011;42:569–583. - PubMed
- Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, et al. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources