Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer - PubMed (original) (raw)
Review
Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer
Charles G Drake et al. Nat Rev Clin Oncol. 2014 Jan.
Abstract
Previously, clinical approaches to using the immune system against cancer focused on vaccines that intended to specifically initiate or amplify a host response against evolving tumours. Although vaccine approaches have had some clinical success, most cancer vaccines fail to induce objective tumour shrinkage in patients. More-recent approaches have centred on a series of molecules known as immune checkpoints-whose natural function is to restrain or dampen a potentially over-exuberant response. Blocking immune checkpoint molecules with monoclonal antibodies has emerged as a viable clinical strategy that mediates tumour shrinkage in several cancer types. In addition to being part of the current treatment armamentarium for metastatic melanoma, immune checkpoint blockade is currently undergoing phase III testing in several cancer types.
Figures
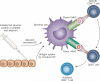
Figure 1
Mechanism of action of cancer vaccines. Cancer vaccines work by providing a target antigen or antigens to a specialized cell known as the dendritic cell (DC). These cells reside at the site of antigen injection (usually intradermal), where they take up and process antigen. Immunostimulatory molecules in the vaccine preparation (adjuvants) activate DCs, which respond by upregulating the molecules they need to interact with (T cells), and migrating to a lymph node. Once in a lymph node, activated DCs present antigen to T cells; if the T cell recognizes its cognate antigen in the proper context, it is then activated. Upon activation, CD4+ T cells produce cytokines that help CD8 T cells to fully mature. Upon full maturation, CD8+ T cells in turn proliferate and then leave the lymph node to traffic widely throughout the body. When an activated T cell senses a cell bearing its target antigen (tumour antigen) in the periphery, it can then lyse that cell, potentially mediating an antitumour response.
Figure 2
Immune checkpoint blockade. This approach to immunotherapy is exemplified by antibodies directed against CTLA-4 (ipilimumab, tremilimumab), which block the immunosupression mediated by the interaction between B7 family members (on antigen-presenting cells) and CTLA-4 (on CD8+ and CD4+ T cells). A second major checkpoint, mediated by the interaction between PD-1 on T cells and its ligand PD-L1 on either antigen-presenting cells or tumour cells, has been the subject of several recent clinical trials, and has shown evidence of efficacy in both non-small-cell lung cancer and renal cell carcinoma.
Comment in
- Reply: Regulatory T cells-an important target for cancer immunotherapy.
Drake CG, Lipson EJ, Brahmer JR. Drake CG, et al. Nat Rev Clin Oncol. 2014 Jun;11(6):307. doi: 10.1038/nrclinonc.2013.208-c2. Epub 2014 Apr 29. Nat Rev Clin Oncol. 2014. PMID: 24781414 Free PMC article. No abstract available. - Regulatory T cells-an important target for cancer immunotherapy.
Shin JI, Ha SJ. Shin JI, et al. Nat Rev Clin Oncol. 2014 Jun;11(6):307. doi: 10.1038/nrclinonc.2013.208-c1. Epub 2014 Apr 29. Nat Rev Clin Oncol. 2014. PMID: 24781417 No abstract available.
Similar articles
- Regulatory T cells-an important target for cancer immunotherapy.
Shin JI, Ha SJ. Shin JI, et al. Nat Rev Clin Oncol. 2014 Jun;11(6):307. doi: 10.1038/nrclinonc.2013.208-c1. Epub 2014 Apr 29. Nat Rev Clin Oncol. 2014. PMID: 24781417 No abstract available. - Reply: Regulatory T cells-an important target for cancer immunotherapy.
Drake CG, Lipson EJ, Brahmer JR. Drake CG, et al. Nat Rev Clin Oncol. 2014 Jun;11(6):307. doi: 10.1038/nrclinonc.2013.208-c2. Epub 2014 Apr 29. Nat Rev Clin Oncol. 2014. PMID: 24781414 Free PMC article. No abstract available. - Prediction of response to anticancer immunotherapy using gene signatures.
Wang E, Bedognetti D, Marincola FM. Wang E, et al. J Clin Oncol. 2013 Jul 1;31(19):2369-71. doi: 10.1200/JCO.2013.49.2157. Epub 2013 May 28. J Clin Oncol. 2013. PMID: 23715576 No abstract available. - Upcoming innovations in lung cancer immunotherapy: focus on immune checkpoint inhibitors.
Aspeslagh S, Marabelle A, Soria JC, Armand JP. Aspeslagh S, et al. Chin Clin Oncol. 2015 Dec;4(4):48. doi: 10.3978/j.issn.2304-3865.2015.12.06. Chin Clin Oncol. 2015. PMID: 26730760 Review. - Lung cancer: potential targets for immunotherapy.
Tartour E, Zitvogel L. Tartour E, et al. Lancet Respir Med. 2013 Sep;1(7):551-63. doi: 10.1016/S2213-2600(13)70159-0. Epub 2013 Aug 23. Lancet Respir Med. 2013. PMID: 24461616 Review.
Cited by
- First-Line Nivolumab and Relatlimab Plus Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma: The Phase II RELATIVITY-060 Study.
Hegewisch-Becker S, Mendez G, Chao J, Nemecek R, Feeney K, Van Cutsem E, Al-Batran SE, Mansoor W, Maisey N, Pazo Cid R, Burge M, Perez-Callejo D, Hipkin RW, Mukherjee S, Lei M, Tang H, Suryawanshi S, Kelly RJ, Tebbutt NC. Hegewisch-Becker S, et al. J Clin Oncol. 2024 Jun 10;42(17):2080-2093. doi: 10.1200/JCO.23.01636. Epub 2024 May 9. J Clin Oncol. 2024. PMID: 38723227 Free PMC article. Clinical Trial. - Fourteen years of cellular deconvolution: methodology, applications, technical evaluation and outstanding challenges.
Nguyen H, Nguyen H, Tran D, Draghici S, Nguyen T. Nguyen H, et al. Nucleic Acids Res. 2024 May 22;52(9):4761-4783. doi: 10.1093/nar/gkae267. Nucleic Acids Res. 2024. PMID: 38619038 Free PMC article. Review. - Informing immunotherapy with multi-omics driven machine learning.
Li Y, Wu X, Fang D, Luo Y. Li Y, et al. NPJ Digit Med. 2024 Mar 14;7(1):67. doi: 10.1038/s41746-024-01043-6. NPJ Digit Med. 2024. PMID: 38486092 Free PMC article. Review. - Establishment of immune suppression by cancer cells in the tumor microenvironment.
Nishikawa H. Nishikawa H. Proc Jpn Acad Ser B Phys Biol Sci. 2024;100(2):114-122. doi: 10.2183/pjab.100.005. Proc Jpn Acad Ser B Phys Biol Sci. 2024. PMID: 38346752 Free PMC article. - Triple-Negative Breast Cancer Intrinsic FTSJ1 Favors Tumor Progression and Attenuates CD8+ T Cell Infiltration.
Sun Y, Liu Q, Zhong S, Wei R, Luo JL. Sun Y, et al. Cancers (Basel). 2024 Jan 31;16(3):597. doi: 10.3390/cancers16030597. Cancers (Basel). 2024. PMID: 38339348 Free PMC article.
References
- McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer. 2009;115:2298–2305. - PubMed
- Escudier B. Emerging immunotherapies for renal cell carcinoma. Ann. Oncol. 2012;23(Suppl. 8):viii35–viii40. - PubMed
- Biswas S, Eisen T. Immunotherapeutic strategies in kidney cancer-when TKIs are not enough. Nat. Rev. Clin. Oncol. 2009;6:478–487. - PubMed
- Nakano O, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA058236/CA/NCI NIH HHS/United States
- R01 CA127153/CA/NCI NIH HHS/United States
- 1P50CA58236‑15/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- T32 CA009071/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical