Measurement of three-dimensional anisotropic diffusion by multiphoton fluorescence recovery after photobleaching - PubMed (original) (raw)
Measurement of three-dimensional anisotropic diffusion by multiphoton fluorescence recovery after photobleaching
Changcheng Shi et al. Ann Biomed Eng. 2014 Mar.
Abstract
The multiphoton fluorescence recovery after photobleaching (MP-FRAP) technique has been developed to measure the three-dimensional (3D) solute diffusion within biological systems. However, current 3D MP-FRAP models are based on isotropic diffusion and spatial domain analysis. The 3D anisotropic diffusion and frequency domain analysis for MP-FRAP measurements are rarely studied. In this study, a new technique is demonstrated for the quantitative and non-destructive determination of 3D anisotropic solute diffusion tensors within biological fibrosis tissues by multiphoton photobleaching and spatial Fourier analysis (SFA). Compared to the spatial domain analysis based MP-FRAP techniques, this SFA-based method has the capability for determining the 3D anisotropic diffusion tensors as well as the flexibility for satisfying initial and boundary conditions. First, a close-form solution of the 3D anisotropic diffusion equation is derived by solely using SFA. Next, this new method is validated by computer-simulated MP-FRAP experiments with pre-defined 3D anisotropic diffusion tensors as well as experimental diffusion measurements of FITC-Dextran (FD) molecules in aqueous glycerol solutions. Finally, this MP-FRAP technique is applied to the measurement of 3D anisotropic diffusion tensors of FD molecules within porcine tendon tissues. This study provides a new tool for complete determination of 3D anisotropic solute diffusion tensor in biological tissues.
Conflict of interest statement
CONFLICT OF INTEREST
None of the authors of this paper have a conflict of interest that might be construed as affecting the conduct or reporting of the work presented.
Figures
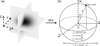
Figure 1
An illustration of the SFA and averaging the diffusivity in the frequency domain over a shell. In the SFA, the solute concentration distribution is transformed from the spatial domain (a) to the frequency domain (b) by using the Fourier transform. In the frequency domain, the diffusivity D(u,v,w) is averaged over a shell of a spherical surface to obtain all components of the 3D diffusion tensor. The level of shell is defined by the radius a (i.e., commonly positive integers) and the range of shell is determined by azimuthal angle φ and polar angle θ.
Figure 2
Two observation protocols for fluorescent solute diffusion measurements in the porcine tendon tissues. X-Y coordinates represent the microscopy coordinates fixed on the microscope sample stage. (a) In the protocol of X-Alignment, the main fiber orientation is aligned with the X-axis. (b) In the protocol of Y-Alignment, the main fiber orientation is aligned with the Y-axis.
Figure 3
The 3D isotropic diffusion of FD500 molecule in glycerol/PBS solutions. (a) Typical time series of 3D MP-FRAP Z-stack image of fluorescent solutes for the experimental diffusion measurements in the glycerol/PBS solutions. For each experiment, typically, 40 stacks of post-bleaching images, plus 1 stack prior to photobleaching, are acquired at an approximate rate of 18 seconds/stack. The central region of the observation volume is photobleached by the multiphoton laser and fluorescence recovers completely in the last Z-stack image series (t=720s). (b) The results of 3D diffusion tensors (mean±SD). In each concentration group, there were no significant differences between the diagonal diffusion components (i.e., Dxx, Dyy, and Dzz), while the off-diagonal diffusion components were significantly smaller than the diagonal diffusion components (p<0.0001). Additionally, the nominal diffusivities (tr(D)/3) of FD500 significantly decreased (ANOVA, p<0.0001) with the increasing glycerol concentrations.
Figure 3
The 3D isotropic diffusion of FD500 molecule in glycerol/PBS solutions. (a) Typical time series of 3D MP-FRAP Z-stack image of fluorescent solutes for the experimental diffusion measurements in the glycerol/PBS solutions. For each experiment, typically, 40 stacks of post-bleaching images, plus 1 stack prior to photobleaching, are acquired at an approximate rate of 18 seconds/stack. The central region of the observation volume is photobleached by the multiphoton laser and fluorescence recovers completely in the last Z-stack image series (t=720s). (b) The results of 3D diffusion tensors (mean±SD). In each concentration group, there were no significant differences between the diagonal diffusion components (i.e., Dxx, Dyy, and Dzz), while the off-diagonal diffusion components were significantly smaller than the diagonal diffusion components (p<0.0001). Additionally, the nominal diffusivities (tr(D)/3) of FD500 significantly decreased (ANOVA, p<0.0001) with the increasing glycerol concentrations.
Figure 4
The 3D anisotropic diffusion of FD molecules in the porcine tendon tissues. (a) Typical time series of 3D MP-FRAP Z-stack image of fluorescent solutes for the experimental diffusion measurements in the porcine tendon tissues. For each experiment, typically, 40 stacks of post-bleaching images, plus 1 stack prior to photobleaching, are acquired at an approximate rate of 18 seconds/stack. The central region of the observation volume is photobleached by the multiphoton laser and fluorescence recovers completely in the last Z-stack image series (t=720s). (b) The results of 3D diffusion tensors (mean±SD). The diffusion of FD70 and FD150 along the major fiber orientation was significantly faster than the other two diffusions transverse to the fiber direction in both the X- and Y-Alignment observation protocols.
Figure 4
The 3D anisotropic diffusion of FD molecules in the porcine tendon tissues. (a) Typical time series of 3D MP-FRAP Z-stack image of fluorescent solutes for the experimental diffusion measurements in the porcine tendon tissues. For each experiment, typically, 40 stacks of post-bleaching images, plus 1 stack prior to photobleaching, are acquired at an approximate rate of 18 seconds/stack. The central region of the observation volume is photobleached by the multiphoton laser and fluorescence recovers completely in the last Z-stack image series (t=720s). (b) The results of 3D diffusion tensors (mean±SD). The diffusion of FD70 and FD150 along the major fiber orientation was significantly faster than the other two diffusions transverse to the fiber direction in both the X- and Y-Alignment observation protocols.
Similar articles
- A noninvasive fluorescence imaging-based platform measures 3D anisotropic extracellular diffusion.
Chen P, Chen X, Hepfer RG, Damon BJ, Shi C, Yao JJ, Coombs MC, Kern MJ, Ye T, Yao H. Chen P, et al. Nat Commun. 2021 Mar 26;12(1):1913. doi: 10.1038/s41467-021-22221-0. Nat Commun. 2021. PMID: 33772014 Free PMC article. - Fluorescence recovery after photobleaching: direct measurement of diffusion anisotropy.
Hashlamoun K, Abusara Z, Ramírez-Torres A, Grillo A, Herzog W, Federico S. Hashlamoun K, et al. Biomech Model Mechanobiol. 2020 Dec;19(6):2397-2412. doi: 10.1007/s10237-020-01346-z. Epub 2020 Jun 19. Biomech Model Mechanobiol. 2020. PMID: 32562093 - Anisotropic solute diffusion tensor in porcine TMJ discs measured by FRAP with spatial Fourier analysis.
Shi C, Kuo J, Bell PD, Yao H. Shi C, et al. Ann Biomed Eng. 2010 Nov;38(11):3398-408. doi: 10.1007/s10439-010-0099-y. Epub 2010 Jun 26. Ann Biomed Eng. 2010. PMID: 20582475 Free PMC article. - The Utility of Fluorescence Recovery after Photobleaching (FRAP) to Study the Plasma Membrane.
Day CA, Kang M. Day CA, et al. Membranes (Basel). 2023 May 2;13(5):492. doi: 10.3390/membranes13050492. Membranes (Basel). 2023. PMID: 37233553 Free PMC article. Review. - Fluorescence recovery after photobleaching: application to nuclear proteins.
Houtsmuller AB. Houtsmuller AB. Adv Biochem Eng Biotechnol. 2005;95:177-99. doi: 10.1007/b102214. Adv Biochem Eng Biotechnol. 2005. PMID: 16080269 Review.
Cited by
- Expanding the applicability of multiphoton fluorescence recovery after photobleaching by incorporating shear stress in laminar flow.
Elias TM, Brown EB Jr, Brown EB 3rd. Elias TM, et al. J Biomed Opt. 2023 Jul;28(7):076502. doi: 10.1117/1.JBO.28.7.076502. Epub 2023 Jul 21. J Biomed Opt. 2023. PMID: 37484975 Free PMC article. - Dissecting protein reaction dynamics in living cells by fluorescence recovery after photobleaching.
Fritzsche M, Charras G. Fritzsche M, et al. Nat Protoc. 2015 May;10(5):660-80. doi: 10.1038/nprot.2015.042. Epub 2015 Apr 2. Nat Protoc. 2015. PMID: 25837418 - A noninvasive fluorescence imaging-based platform measures 3D anisotropic extracellular diffusion.
Chen P, Chen X, Hepfer RG, Damon BJ, Shi C, Yao JJ, Coombs MC, Kern MJ, Ye T, Yao H. Chen P, et al. Nat Commun. 2021 Mar 26;12(1):1913. doi: 10.1038/s41467-021-22221-0. Nat Commun. 2021. PMID: 33772014 Free PMC article. - Inhibition of transglutaminase activity in periodontitis rescues periodontal ligament collagen content and architecture.
Moore Rosset E, Trombetta-eSilva J, Hepfer G, Chen P, Yao H, Bradshaw AD. Moore Rosset E, et al. J Periodontal Res. 2020 Jan;55(1):107-115. doi: 10.1111/jre.12694. Epub 2019 Sep 25. J Periodontal Res. 2020. PMID: 31552683 Free PMC article. - Toward dynamic, anisotropic, high-resolution, and functional measurement in the brain extracellular space.
Xu X, Ge X, Xiong H, Qin Z. Xu X, et al. Neurophotonics. 2022 Jul;9(3):032210. doi: 10.1117/1.NPh.9.3.032210. Epub 2022 May 11. Neurophotonics. 2022. PMID: 35573823 Free PMC article. Review.
References
- Abrahamsson S, Chen J, Hajj B, Stallinga S, Katsov AY, Wisniewski J, Mizuguchi G, Soule P, Mueller F, Dugast Darzacq C, Darzacq X, Wu C, Bargmann CI, Agard DA, Dahan M, Gustafsson MG. Fast multicolor 3D imaging using aberration-corrected multifocus microscopy. Nat Methods. 2013;10:60–63. - PMC - PubMed
- Brown EB, Boucher Y, Nasser S, Jain RK. Measurement of macromolecular diffusion coefficients in human tumors. Microvasc. Res. 2004;67:231–236. - PubMed
Publication types
MeSH terms
Grants and funding
- R03 DE018741/DE/NIDCR NIH HHS/United States
- R03 AR055775/AR/NIAMS NIH HHS/United States
- DE021134/DE/NIDCR NIH HHS/United States
- P20 RR017696/RR/NCRR NIH HHS/United States
- R01 DE021134/DE/NIDCR NIH HHS/United States
- DE018741/DE/NIDCR NIH HHS/United States
- AR055775/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous