Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse - PubMed (original) (raw)
Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse
Michele Salanova et al. Redox Biol. 2013.
Abstract
Activity-induced nitric oxide (NO) imbalance and "nitrosative stress" are proposed mechanisms of disrupted Ca(2+) homeostasis in atrophic skeletal muscle. We thus mapped S-nitrosylated (SNO) functional muscle proteins in healthy male subjects in a long-term bed rest study (BBR2-2 Study) without and with exercise as countermeasure in order to assess (i) the negative effects of chronic muscle disuse by nitrosative stress, (ii) to test for possible attenuation by exercise countermeasure in bed rest and (iii) to identify new NO target proteins. Muscle biopsies from calf soleus and hip vastus lateralis were harvested at start (Pre) and at end (End) from a bed rest disuse control group (CTR, n=9) and two bed rest resistive exercise groups either without (RE, n=7) or with superimposed vibration stimuli (RVE, n=7). At subcellular compartments, strong anti-SNO-Cys immunofluorescence patterns in control muscle fibers after bed rest returned to baseline following vibration exercise. Total SNO-protein levels, Nrf-2 gene expression and nucleocytoplasmic shuttling were changed to varying degrees in all groups. Excess SNO-protein levels of specific calcium release/uptake proteins (SNO-RyR1, -SERCA1 and -PMCA) and of contractile myosin heavy chains seen in biopsy samples of chronically disused skeletal muscle were largely reduced by vibration exercise. We also identified NOS1 as a novel NO target in human skeletal muscle controlled by activity driven auto-nitrosylation mechanisms. Our findings suggest that aberrant levels of functional SNO-proteins represent signatures of uncontrolled nitrosative stress management in disused human skeletal muscle that can be offset by exercise as countermeasure.
Keywords: Calcium ATPase; Calcium homeostasis; Calcium-release channels; Nitric oxide synthase; Nitrosative stress; Nrf-2; Skeletal muscle.
Figures
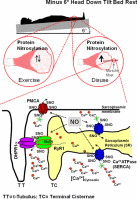
Graphical abstract
Fig. 1
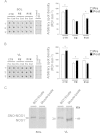
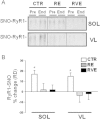
SNO-protein assay in human skeletal muscle biopsies. A. Dot blots analysis (left panel) biotin-labeled proteins (reflecting SNO-proteins) in soleus (SOL) of End vs. Pre bed rest subjects (1,2,3: triplicate), Control BST was obtained by omitting biotin-HPDP during the protocol; right panel, dot blot densitometry analysis. B. (left panel) Dot blot analysis of biotin-labeled proteins in vastus lateralis (VL) of End vs. Pre bed rest subjects (1,2,3: triplicate), Control BST was obtained by omitting biotin-HPDP during the protocol; right panel, dot blot densitometry analysis. A significant increase of biotin incorporation is observed in CTR group (_n_=9) in both muscles (SOL, Pre 86.51±2.9, End 95.64±6.5; VL Pre 192.94±3.07, End 222.59±6.6). In the RE group (_n_=7) a significant increase was in VL only (Pre 123.07±4.7, End 145.6±5.56) but not in SOL after bed rest. RVE (_n_=7) only significantly decreased SNO-protein levels in both SOL (Pre 75.3±0.75, End 62.25±1.7) and VL (Pre 144.52±3.8, End 122.32±4.1). C. NOS1 WB analysis of BST streptavidin column eluate vs. normal muscle lysates (Muscle lysate). In both SOL and VL, NOS1 immunoreactive bands with higher relative mobility are detectable (BST/Eluate) vs. a predicted NOS1 immunoreactive band obtained from total muscle lysates used as positive control.
Fig. 2
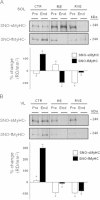
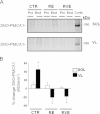
BST-related SNO-NOS1 protein assay of human SOL and VL muscle biopsies before and after bed rest. A. Upper panel, NOS1 WB analysis of SOL lysates normalized to alpha tubulin. A. Middle panel, NOS1 WB analysis of BST-streptavidin column eluate in SOL lysates. NOS1 protein immunoreactive bands reflecting biotin-labeled NOS1 proteins were present in all eluate samples showing the presence of S-nitrosylated NOS1 proteins in human SOL. A. Lower panel, graph representing percent changes of NOS1 (white columns, CTR (_n_=9), −32%, _p_≤0.01; RE (_n_=7), 12.7%, _p_≤0.05; RVE (_n_=7), 31.2%, _p_≤0.01) and of SNO-NOS1 (black columns, CTR (_n_=9), −40%, _p_≤0.01; RE (_n_=7), 42.5%, _p_≤0.01; RVE (_n_=7), 60.6%, _p_≤0.01) proteins in SOL of End vs. Pre bed rest biopsies (Pre values are set up as zero baselines). B. Upper panel, NOS1 WB analysis of VL lysates normalized to alpha tubulin. B. Middle panel, NOS1 WB analysis of BST-streptavidin column eluate in VL lysates. NOS1 protein immunoreactive bands reflecting biotin-labeled NOS1 proteins were present in all eluate samples showing the presence of S-nitrosylated NOS1 proteins in human VL. B. Lower panel, graph representing percent changes of NOS1 (white columns, CTR (_n_=9), −9.84%, _p_≤0.05; RE (_n_=7), 16.2%, _p_≤0.01; RVE (_n_=7), 9.5%, _p_≤0.05) and of SNO-NOS1 (black columns, CTR (_n_=9), −12%, _p_≤0.05; RE (_n_=7), 10.45%, _p_≤0.05; RVE (_n_=7), 15.65%, _p_≤0.01) proteins in VL lysates of End vs. Pre bed rest samples (Pre values are set up as zero baselines).
Fig. 3
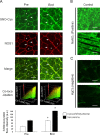
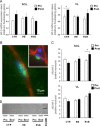
Confocal S-nitrosocystein (SNO-Cys) immunohistochemistry analysis in human skeletal muscle biopsies. A. Before start of bed rest (Pre) anti-SNO-Cys, anti-NOS1, and merged images (Merge). Anti-SNO-Cys antibodies immunolabelled the subsarcolemmal compartment (arrows, green fluorescence channel) that almost fully overlapped with anti-NOS1 antibodies immunostaining, red channel immunofluorescence signals (red and green merged yellow pixel area in 3D co-localization box plot at bottom panel), the cytosol/sarcoplasmic reticulum (SR)/myofibrillar compartment (asterisks). After bed rest (End), significantly increased SNO-Cys immunofluorescence was seen in SOL. A. Lower panel, graph representing anti-SNO-Cys immunosignal intensity in CTR Pre and End bed rest SOL. Significant changes in NOS1 (not shown) and SNO-Cys proteins were detected by intracellular pixel area intensity analysis (CTR (_n_=5) Pre, 36.55, _p_≤0.01; CTR (_n_=5) End, 75.55, _p_≤0.01) and sarcolemmal (CTR (_n_=5) Pre, 77.78, _p_≤0.01; CTR (_n_=5) End, 143.89, _p_≤0.01). B. Positive control. Cryosection was pre-incubated with NaNO2 (producing nitroso compounds) followed by SNO-Cys immunostaining. C. Negative control. Cryosection was blocked by pre-incubation with HgCl2 followed by anti-SNO-Cys immunostaining. Bar=50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4
BST detection of SNO-myosin heavy chain (MyHC) proteins in bed rest muscle biopsies. A. Immunoblotted BST streptavidin column eluate of SOL investigated for the presence of fast- (upper panel) and slow-type (lower panel) MyHC. Significant decrease in slow-type (−22%, _p_≤0.05) and increase in fast-type (138%, _p_≤0.01) MyHC was present in CTR (_n_=9) End bed rest biopsies. In the RE group (_n_=7), an increase of slow-type (51%, _p_≤0.01) and a decrease of fast-type (−99%, _p_≤0.01) MyHC was seen. In the RVE (_n_=4) group, both slow-type (−99%, _p_≤0.01) and fast-type (−16%, _p_≤0.05) MyHCs levels were equally decreased. B. Slow- and fast-type MyHC (s/fMyHC) immunoblot analysis of BST streptavidin column eluate of VL. Significant increase of slow-type (13%, _p_≤0.05, lower panel) and fast-type (300%, _p_≤0.01) MyHCs were present in CTR (_n_=9) End bed rest biopsies. In the RE group (_n_=7), a decrease of slow-type (−92%, _p_≤0.01) and fast-type (−15%, _p_≤0.05) MyHC was present. In the RVE group (_n_=7), both slow- (−95%, _p_≤0.01) and fast-type (−80%, _p_≤0.01) MyHC levels were equally decreased.
Fig. 5
BST detection of SNO-RyR1 in human skeletal muscle SOL and VL biopsies. A. Upper panel, Immunoblot of BST streptavidin column eluate of SOL for RyR1. A. Lower panel, Immunoblot of BST streptavidin column eluate of VL for RyR1. Increased RyR1 immunoreactive band was detected in CTR End-only samples. B. Percent changes biotin-labeled RyR1 proteins from Pre and End bed rest biopsies. Bed rest without exercise promoted _S_-nitrosylation of RyR1 in disused SOL (CTR _n_=9, 18.6%, _p_≤0.01) and VL (CTR _n_=9, 17.2%, _p_≤0.01) as counteracted by both exercise countermeasures. (* significance).
Fig. 6
BST detection of sarcoplasmic reticulum SNO-SERCA1/–SERCA2 in human skeletal muscle SOL and VL biopsies from bed rest groups. A. Upper panel, Immunoblot of SERCA1 in BST streptavidin column eluate of SOL; lower panel, percent changes SNO-Cys-SERCA1 and SNO-Cys-SERCA2 of End vs. Pre bed rest SOL (Pre values are set up as zero baselines). An increase of SNO-SERCA1 in CTR (330%, _p_≤0.01, _n_=9), RE (180%, _p_≤0.01, _n_=7), and RVE (75%, _p_≤0.01, _n_=7) group was detected (*). No changes were observed for SNO-SERCA2 proteins. B. Upper panel, Immunoblot of SERCA1 of streptavidin column eluate of VL; lower panel, percent changes SNO-Cys-SERCA1 and SNO-Cys-SERCA2 of End vs. Pre bed rest VL (Pre values are set up as zero baselines). An increase of SERCA1 (220%, _p_≤0.01, _n_=9) was present in CTR End subjects, while a decrease was present in RE (-41%, _p_≤0.01, _n_=7) and RVE (-40%, _p_≤0.01, _n_=7) subjects (*). No changes were observed for SNO-SERCA2 proteins.
Fig. 7
BST detection of sarcolemmal and sarcoplasmatic reticulum functional channel proteins in human skeletal SOL and VL from bed rest subjects. A. Upper and lower panel, DHPR1_α_ immunoblot of BST streptavidin column eluate of SOL and VL. A moderate presence of DHPR1_α_ proteins was present in all groups (CTR _n_=9; RE _n_=7; RVE _n_=7). B. Upper and lower panel, IP3R1 immunoblot of BST streptavidin column eluate of SOL and VL. A moderate presence of IP3R1 proteins was present in all groups (Pre). No changes were present in both samples after bed rest (End) (CTR _n_=9; RE _n_=7; RVE _n_=7). C. Left and right panel, TRPC1 immunoblot of BST streptavidin column eluate of SOL and VL (CTR _n_=9; RE _n_=7; RVE _n_=7); (Contr.=positive control of total proteins muscle lysates). No SNO-TRPC1 protein signals were detectable in either group before and after bed rest.
Fig. 8
BST detection of sarcoplasmic membrane SNO-PMCA1 in human skeletal muscle SOL and VL from bed rest groups. A. Upper panel, PMCA1 immunoblot BST streptavidin column eluate of SOL (CTR _n_=9; RE _n_=7; RVE _n_=7); a faint PMCA1 immunoreactive band was present in all groups. A. Lower panel, PMCA1 immunoblot of streptavidin column eluate of VL (CTR _n_=9; RE _n_=7; RVE _n_=7); a faint PMCA1 immunoreactive band was present in all groups. Control=positive control with human endothelial cell lysates (BD Biosciences). B. Percent change SNO-PMCA1 in SOL and VL of all groups. No changes in SNO-PMCA1 were found SOL (CTR 9.4%, _p_>0.05; RE SOL 7.5%, _p_>0.05, RVE 1.78%, _p_>0.05) while changes were present in VL (CTR 45.55%, _p_≤0.01; RE −21.35%, _p_≤0.01; RVE −19.07%, _p_≤0.01) after bed rest (* significance).
Fig. 9
Anti-oxidative master gene Nrf-2 regulation in SOL and VL of bed rest subjects. A. Quantitative PCR analysis with Nrf-2 specific probe in SOL (left panel) and VL (right panel). Increase in Nrf-2 transcripts was detected after bed rest only in CTR group in VL (+39.33%, *_P_=0.016). B. Nrf-2 immunopositive myonucleus (green) in SOL after bed rest. Dystrophin (red) was used to label the subsarcolemmal border; nuclei were counterstained with DAPI (blue). Inset, positive Nrf-2 myonucleus for comparison with Nrf-2 negative nuclei outside the myofiber. C. Quantitative analysis of Nrf-2 positive myonuclei in SOL (upper panel) and VL (lower panel). The total number of Nrf-2 positive myonuclei was slightly reduced in both, SOL (Pre, 10.93±0.66%, End, 9.03±0.75%) and VL (Pre, 9.83±0.71%, End, 8.33±0.86%) of CTR group after bed rest. In the RE group no changes were seen in SOL (Pre, 9.62±0.77%, End, 11.25±1.19%) and VL (Pre, 8.77±0.54%, End, 9.39±0.54%). In RVE group a increased numbers of Nrf-2 positive myonuclei were present in SOL (+30.55%, *_P_=0.012) but absent in VL (+17%, _P_=0.15). D. Nrf-2 immunoblot of VL muscle lysates in all groups. A faint Nrf-2 immunoreactive band with an apparent MW similar to the positive control (contr.) was detected in all samples. Contr.: Jurkat whole cell lysates (SC-2204, Santa Cruz Biotechnology, CA). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Similar articles
- Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures.
Salanova M, Schiffl G, Püttmann B, Schoser BG, Blottner D. Salanova M, et al. J Anat. 2008 Mar;212(3):306-18. doi: 10.1111/j.1469-7580.2008.00854.x. Epub 2008 Jan 21. J Anat. 2008. PMID: 18221329 Free PMC article. Clinical Trial. - Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure.
Salanova M, Schiffl G, Rittweger J, Felsenberg D, Blottner D. Salanova M, et al. Histochem Cell Biol. 2008 Jul;130(1):105-18. doi: 10.1007/s00418-008-0399-6. Epub 2008 Feb 19. Histochem Cell Biol. 2008. PMID: 18283481 - Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest.
Blottner D, Salanova M, Püttmann B, Schiffl G, Felsenberg D, Buehring B, Rittweger J. Blottner D, et al. Eur J Appl Physiol. 2006 Jun;97(3):261-71. doi: 10.1007/s00421-006-0160-6. Epub 2006 Mar 28. Eur J Appl Physiol. 2006. PMID: 16568340 Clinical Trial. - Glutathione and Nitric Oxide: Key Team Players in Use and Disuse of Skeletal Muscle.
Baldelli S, Ciccarone F, Limongi D, Checconi P, Palamara AT, Ciriolo MR. Baldelli S, et al. Nutrients. 2019 Sep 30;11(10):2318. doi: 10.3390/nu11102318. Nutrients. 2019. PMID: 31575008 Free PMC article. Review. - Determinants of disuse-induced skeletal muscle atrophy: exercise and nutrition countermeasures to prevent protein loss.
Bajotto G, Shimomura Y. Bajotto G, et al. J Nutr Sci Vitaminol (Tokyo). 2006 Aug;52(4):233-47. doi: 10.3177/jnsv.52.233. J Nutr Sci Vitaminol (Tokyo). 2006. PMID: 17087049 Review.
Cited by
- Nrf2 mediates redox adaptations to exercise.
Done AJ, Traustadóttir T. Done AJ, et al. Redox Biol. 2016 Dec;10:191-199. doi: 10.1016/j.redox.2016.10.003. Epub 2016 Oct 14. Redox Biol. 2016. PMID: 27770706 Free PMC article. Review. - Vibration mechanosignals superimposed to resistive exercise result in baseline skeletal muscle transcriptome profiles following chronic disuse in bed rest.
Salanova M, Gambara G, Moriggi M, Vasso M, Ungethuem U, Belavý DL, Felsenberg D, Cerretelli P, Gelfi C, Blottner D. Salanova M, et al. Sci Rep. 2015 Nov 24;5:17027. doi: 10.1038/srep17027. Sci Rep. 2015. PMID: 26596638 Free PMC article. - Spaceflight on the ISS changed the skeletal muscle proteome of two astronauts.
Murgia M, Rittweger J, Reggiani C, Bottinelli R, Mann M, Schiaffino S, Narici MV. Murgia M, et al. NPJ Microgravity. 2024 Jun 5;10(1):60. doi: 10.1038/s41526-024-00406-3. NPJ Microgravity. 2024. PMID: 38839773 Free PMC article. - nNOS-derived NO modulates force production and iNO-derived NO the excitability in C2C12-derived 3D tissue engineering skeletal muscle via different NO signaling pathways.
Mosqueira M, Scheid LM, Kiemel D, Richardt T, Rheinberger M, Ollech D, Lutge A, Heißenberg T, Pfitzer L, Engelskircher L, Yildiz U, Porth I. Mosqueira M, et al. Front Physiol. 2022 Aug 15;13:946682. doi: 10.3389/fphys.2022.946682. eCollection 2022. Front Physiol. 2022. PMID: 36045747 Free PMC article. - Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity.
Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, Guise TA, Rubin J, Rubin CT. Pagnotti GM, et al. Nat Rev Endocrinol. 2019 Jun;15(6):339-355. doi: 10.1038/s41574-019-0170-1. Nat Rev Endocrinol. 2019. PMID: 30814687 Free PMC article. Review.
References
- Powers S.K., Kavazis A.N., DeRuisseau K.C. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R337–R344. - PubMed
- Kashiba-Iwatsuki M., Kitoh K., Kasahara E., Yu H., Nisikawa M., Matsuo M., Inoue M. Ascorbic acid and reducing agents regulate the fates and functions of S-nitrosothiols. J. Biochem. 1997;122:1208–1214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous