Eyeballing cholesterol efflux and macrophage function in disease pathogenesis - PubMed (original) (raw)
Review
Eyeballing cholesterol efflux and macrophage function in disease pathogenesis
Abdoulaye Sene et al. Trends Endocrinol Metab. 2014 Mar.
Abstract
Disorders of lipid metabolism are strongly associated with cardiovascular disease. Recently, there has been significant focus on how tissues process lipid deposits. Impaired cholesterol efflux has been shown to be crucial in mediating lipid deposition in atherosclerosis. The inability of macrophages to effectively efflux cholesterol from tissues initiates inflammation, plaque neovascularization, and subsequent rupture. Recent studies suggest that inability to effectively efflux cholesterol from tissues may have global implications far beyond atherosclerosis, extending to the pathophysiology of unrelated diseases. We examine the unifying mechanisms by which impaired cholesterol efflux facilitates tissue-specific inflammation and disease progression in age-related macular degeneration (AMD), a blinding eye disease, and in atherosclerosis, a disease associated with significant cardiovascular morbidity.
Keywords: AMD; atherosclerosis; cholesterol efflux; lipids; macrophage.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
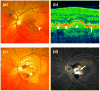
Figure 1. Clinical features of AMD
(a) Fundus photograph of the retina of a patient with dry AMD demonstrates large lipid laden drusen (arrowhead) underneath the retina. (b) Corresponding optical coherent tomography (OCT) of the central retina (macula) confirmed the presence of multiple drusen (arrowheads) underneath the retinal pigment epithelium layer (RPE-arrow). (c) Fundus photograph of a patient with the wet form of AMD illustrate subretinal hemorrhage and fluid (arrowhead) secondary to choroidal neovascularization (CNV, dotted circle). A fluorescein angiogram demonstrates leakage of dye from the CNV (arrow).
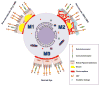
Figure 2. The central role of macrophage-mediated immunity in disease pathogenesis of AMD
Under baseline conditions, non-polarized macrophages (M0) traffic through the choriocapillaris underneath the retina sampling the tissue microenvironment. Aging and complex genetic factors impair macrophage function including reverse cholesterol transport (RCT) and lead to the deposition of lipid rich drusen underneath the retina and RPE in non-neovascular (dry) AMD. In dry AMD, dysfunctional classically activated macrophages (M1) can also induce inflammation driven cell death that leads to advanced stages of dry AMD characterized by loss of RPE cells, called geographic atrophy (GA) and subsequent loss of rod and cone photoreceptor neurons (PR). In the more aggressive form of disease, impaired macrophage RCT in the drusen rich sub-retinal micromilieu polarizes these cells to an alternatively activated (M2) phenotype. Alternatively activated macrophages are disease promoting and pro-angiogenic and facilitate the development of CNV. The sine qua non of wet AMD is the development of CNV, characterized by the growth of new blood vessels from the choroid into the sub-retinal space. These neovascular complexes are leaky and lead to exudation and hemorrhage with disruption of the retinal architecture, interference with the central visual axis, loss of PR and ultimately, irreversible vision loss.
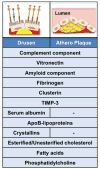
Figure 3. Drusen composition and similarities with atherosclerotic plaques
Analysis of the composition of lipid-rich deposits (yellow) in drusen underneath the retina (left upper panel), or within atherosclerotic plaques (right upper panel) demonstrates similar constituents. Many complement proteins have been identified in drusen and atherosclerotic plaque suggesting an activation of the complement pathway within the deposits. Vitronectin, amyloid component, fibrinogen, clusterin and tissue inhibitor of metalloproteinase 3 (TIMP-3) are molecular constituents of both drusen and atherosclerotic plaques. In contrast, serum albumin and crystallins proteins are exclusive drusen components. ApoB lipoproteins are major constituents of drusen and atherosclerotic plaques. In addition to the plasma, the apoB deposited in drusen also have an intraocular source. Lipid profiling of drusen and atherosclerotic plaques revealed that they both contain esterified and unesterified cholesterol, fatty acids and phosphatidylcholine.
Similar articles
- Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration.
Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS. Sene A, et al. Cell Metab. 2013 Apr 2;17(4):549-61. doi: 10.1016/j.cmet.2013.03.009. Cell Metab. 2013. PMID: 23562078 Free PMC article. - Macrophage Mitochondrial Energy Status Regulates Cholesterol Efflux and Is Enhanced by Anti-miR33 in Atherosclerosis.
Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, Moore KJ, Perisic L, Maegdefessel L, Hedin U, Harper ME, Rayner KJ. Karunakaran D, et al. Circ Res. 2015 Jul 17;117(3):266-78. doi: 10.1161/CIRCRESAHA.117.305624. Epub 2015 May 22. Circ Res. 2015. PMID: 26002865 Free PMC article. - Tanshinone IIA Promotes Macrophage Cholesterol Efflux and Attenuates Atherosclerosis of apoE-/- Mice by Omentin-1/ABCA1 Pathway.
Tan YL, Ou HX, Zhang M, Gong D, Zhao ZW, Chen LY, Xia XD, Mo ZC, Tang CK. Tan YL, et al. Curr Pharm Biotechnol. 2019;20(5):422-432. doi: 10.2174/1389201020666190404125213. Curr Pharm Biotechnol. 2019. PMID: 30947667 - PPARβ in macrophages and atherosclerosis.
Chinetti-Gbaguidi G, Staels B. Chinetti-Gbaguidi G, et al. Biochimie. 2017 May;136:59-64. doi: 10.1016/j.biochi.2016.12.008. Epub 2016 Dec 21. Biochimie. 2017. PMID: 28011212 Review. - The macrophage and its related cholesterol efflux as a HDL function index in atherosclerosis.
Yamamoto S, Narita I, Kotani K. Yamamoto S, et al. Clin Chim Acta. 2016 Jun 1;457:117-22. doi: 10.1016/j.cca.2016.04.012. Epub 2016 Apr 15. Clin Chim Acta. 2016. PMID: 27087419 Review.
Cited by
- Age-Related Macular Degeneration in Patients with Androgenetic Alopecia: Could the Monocyte/HDL Ratio Be the Link?
Shams GM, Saleh AA, Saeed AM, El-Damaty SN, Abdel-Ghaffar AO. Shams GM, et al. Dermatol Pract Concept. 2023 Oct 1;13(4):e2023285. doi: 10.5826/dpc.1304a285. Dermatol Pract Concept. 2023. PMID: 37992380 Free PMC article. - Adult body height and age-related macular degeneration in healthy individuals: A nationwide population-based survey from Korea.
Hwang IC, Bae JH, Kim JM, Lee JM, Nguyen QD. Hwang IC, et al. PLoS One. 2020 May 1;15(5):e0232593. doi: 10.1371/journal.pone.0232593. eCollection 2020. PLoS One. 2020. PMID: 32357183 Free PMC article. - Drusen and pachydrusen: the definition, pathogenesis, and clinical significance.
Zhang X, Sivaprasad S. Zhang X, et al. Eye (Lond). 2021 Jan;35(1):121-133. doi: 10.1038/s41433-020-01265-4. Epub 2020 Nov 18. Eye (Lond). 2021. PMID: 33208847 Free PMC article. Review. - Cholesterol Accumulation Promotes Photoreceptor Senescence and Retinal Degeneration.
Terao R, Sohn BS, Yamamoto T, Lee TJ, Colasanti J, Pfeifer CW, Lin JB, Santeford A, Yamaguchi S, Yoshida M, Apte RS. Terao R, et al. Invest Ophthalmol Vis Sci. 2024 Aug 1;65(10):29. doi: 10.1167/iovs.65.10.29. Invest Ophthalmol Vis Sci. 2024. PMID: 39167399 Free PMC article. - Oxysterol Signatures Distinguish Age-Related Macular Degeneration from Physiologic Aging.
Lin JB, Sene A, Santeford A, Fujiwara H, Sidhu R, Ligon MM, Shankar VA, Ban N, Mysorekar IU, Ory DS, Apte RS. Lin JB, et al. EBioMedicine. 2018 Jun;32:9-20. doi: 10.1016/j.ebiom.2018.05.035. Epub 2018 Jun 11. EBioMedicine. 2018. PMID: 29903570 Free PMC article.
References
- Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Archives of ophthalmology. 2004;122:564–572. - PubMed
- Hageman GS, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Progress in retinal and eye research. 2001;20:705–732. - PubMed
- Zipfel PF, et al. The role of complement in AMD. Advances in experimental medicine and biology. 2010;703:9–24. - PubMed
- Cherepanoff S, et al. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. The British journal of ophthalmology. 2010;94:918–925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EY002687/EY/NEI NIH HHS/United States
- K08 EY016139/EY/NEI NIH HHS/United States
- R01 EY019287/EY/NEI NIH HHS/United States
- K08EY016139/EY/NEI NIH HHS/United States
- P30EY02687/EY/NEI NIH HHS/United States
- R01EY019287/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical