Extensive deamidation at asparagine residue 279 accounts for weak immunoreactivity of tau with RD4 antibody in Alzheimer's disease brain - PubMed (original) (raw)
doi: 10.1186/2051-5960-1-54.
Muneaki Takahashi, Masami Masuda-Suzukake, Fuyuki Kametani, Takashi Nonaka, Hiromi Kondo, Haruhiko Akiyama, Takao Arai, David M A Mann, Yuko Saito, Hiroyuki Hatsuta, Shigeo Murayama, Masato Hasegawa
Affiliations
- PMID: 24252707
- PMCID: PMC3893535
- DOI: 10.1186/2051-5960-1-54
Extensive deamidation at asparagine residue 279 accounts for weak immunoreactivity of tau with RD4 antibody in Alzheimer's disease brain
Ayaho Dan et al. Acta Neuropathol Commun. 2013.
Abstract
Background: Intracytoplasmic inclusions composed of filamentous tau proteins are defining characteristics of neurodegenerative tauopathies, but it remains unclear why different tau isoforms accumulate in different diseases and how they induce abnormal filamentous structures and pathologies. Two tau isoform-specific antibodies, RD3 and RD4, are widely used for immunohistochemical and biochemical studies of tau species in diseased brains.
Results: Here, we show that extensive irreversible post-translational deamidation takes place at asparagine residue 279 (N279) in the RD4 epitope of tau in Alzheimer's disease (AD), but not corticobasal degeneration (CBD) or progressive supranuclear palsy (PSP), and this modification abrogates the immunoreactivity to RD4. An antiserum raised against deamidated RD4 peptide specifically recognized 4R tau isoforms, regardless of deamidation, and strongly stained tau in AD brain. We also found that mutant tau with N279D substitution showed reduced ability to bind to microtubules and to promote microtubule assembly.
Conclusion: The biochemical and structural differences of tau in AD from that in 4R tauopathies found in this study may therefore have implications for prion-like propagation of tau.
Figures
Figure 1
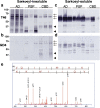
Immunoblot and LC/MS/MS analyses showed a lower immunoreactivity of RD4 with tau in AD and deamidation at N279. Immunoblot analysis of Sarkosyl-insoluble (a, b) and soluble (c, d) tau from AD, PSP and CBD brains (three cases for each disease) with anti-tau monoclonal antibodies T46 (a, c) and RD4 (b, d). Arrows indicated the positions of the 60, 64, 68 kDa triplet tau bands in AD brains, and arrowheads indicate the ~33 and ~37 kDa C-terminal fragments that distinguish PSP and CBD. Identification of deamidated amino acid residue by nano-electrospray tandem mass spectrometry (e). Product ion spectrum of a mass signal of tryptic peptide VQIINK detected in Sarkosyl-insoluble tau from AD brain, showing the b and y ion series. These results identify the site of deamidation as N279, indicated by N*.
Figure 2
RD4 cannot recognize N279D-4R tau, but new anti-4R labels both WT and N279D-4R tau equally. Immunoblot analysis of wild-type (WT) and N279D mutant tau before (WT, N279D) and after (p-WT, p-N279D) phosphorylation with PKA, using T46 (a), RD4 (b), anti-4R (c) and 12E8 (d) antibodies. Immunoblot analysis of six recombinant human tau isoforms with T46, RD3, RD4 and anti-4R antibodies (e). Specificities of RD4 (1:1000 dilutions) and anti-4R (1:3000 dilutions) antibodies for synthetic peptides, L-Asn (wild-type), L-Asp, L-isoAsp and D-Asp peptides (0.625 ~ 10 μg/mL) tested by ELISA assay (f). Quantitation of the ELISA results (the mean of absorbance at 490 nm on 1.25 μg/mL peptide is shown (g). Pathways for deamidation of asparaginyl residues (h). L-Asn residue can be converted spontaneously via a succinimidyl intermediate to form L-Asp, D-Asp. L-isoAsp and D-isoAsp residue (modified from Ref. [9]).
Figure 3
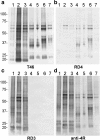
New anti-4R strongly stained tau bands and smears in AD. Immunoblot analyses of Sarkosyl-insoluble tau from three AD (1–3), two PSP(4, 5) and two CBD (6, 7) cases with T46 (a, 1:2000 dilution), RD4 (b, 1:1000 dilution), RD3 (c, 1:1000 dilution) and anti-4R antibodies (d, 1:2000 dilution).
Figure 4
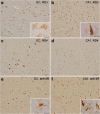
New anti-4R antibody stained intracellular NFTs more extensively than did RD4. Immunostaining of entorhinal cortex (EC) and CA1 sections of AD brain after autoclaving and formic acid treatments, using RD3 (a, b), RD4 (c, d) and anti-4R (e, f) antibodies. NFTs with higher magnification are shown in insets. Bar = 100 μm (25 μm in insets).
Figure 5
Deamidation of N279 reduced the functional activity of tau. Effects of deamidation at N279 on the ability of four repeat htau46 (412 amino acid isoform of human tau) to promote microtubule assembly (a) and to bind to microtubules (b, c). a, Polymerisation of tubulin induced by wild-type htau46 (solid line) and htau46 N279D (dotted line) was monitored by turbidimetry. Results of a typical experiment are shown; similar results were obtained in three separate experiments. b, SDS-PAGE and CBB stainings of free tau and tau bound (arrows) to a constant amount of tubulin (small arrow) are shown. c, Scatter plots of quantitations of free tau and tau bound to WT tau (open circles) and N279D mutant tau (open squares).
Similar articles
- Different immunoreactivities of the microtubule-binding region of tau and its molecular basis in brains from patients with Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration.
Arai T, Ikeda K, Akiyama H, Tsuchiya K, Iritani S, Ishiguro K, Yagishita S, Oda T, Odawara T, Iseki E. Arai T, et al. Acta Neuropathol. 2003 May;105(5):489-98. doi: 10.1007/s00401-003-0671-8. Epub 2003 Feb 8. Acta Neuropathol. 2003. PMID: 12677450 - Tangle evolution linked to differential 3- and 4-repeat tau isoform deposition: a double immunofluorolabeling study using two monoclonal antibodies.
Uchihara T, Hara M, Nakamura A, Hirokawa K. Uchihara T, et al. Histochem Cell Biol. 2012 Feb;137(2):261-7. doi: 10.1007/s00418-011-0891-2. Epub 2011 Nov 25. Histochem Cell Biol. 2012. PMID: 22116524 - Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies.
Yoshida M. Yoshida M. Neuropathology. 2006 Oct;26(5):457-70. doi: 10.1111/j.1440-1789.2006.00743.x. Neuropathology. 2006. PMID: 17080726 - Structure of NFT: Biochemical Approach.
Hasegawa M. Hasegawa M. Adv Exp Med Biol. 2019;1184:23-34. doi: 10.1007/978-981-32-9358-8_2. Adv Exp Med Biol. 2019. PMID: 32096025 Review.
Cited by
- Visualization of neurofibrillary tangle maturity in Alzheimer's disease: A clinicopathologic perspective for biomarker research.
Moloney CM, Lowe VJ, Murray ME. Moloney CM, et al. Alzheimers Dement. 2021 Sep;17(9):1554-1574. doi: 10.1002/alz.12321. Epub 2021 Apr 2. Alzheimers Dement. 2021. PMID: 33797838 Free PMC article. Review. - Cryo-EM structures of tau filaments from Alzheimer's disease.
Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres SHW. Fitzpatrick AWP, et al. Nature. 2017 Jul 13;547(7662):185-190. doi: 10.1038/nature23002. Epub 2017 Jul 5. Nature. 2017. PMID: 28678775 Free PMC article. - 3R and 4R tau isoforms in paired helical filaments in Alzheimer's disease.
Hasegawa M, Watanabe S, Kondo H, Akiyama H, Mann DM, Saito Y, Murayama S. Hasegawa M, et al. Acta Neuropathol. 2014 Feb;127(2):303-5. doi: 10.1007/s00401-013-1191-9. Epub 2013 Nov 9. Acta Neuropathol. 2014. PMID: 24212601 Free PMC article. No abstract available. - Structure of Tau filaments in Prion protein amyloidoses.
Hallinan GI, Hoq MR, Ghosh M, Vago FS, Fernandez A, Garringer HJ, Vidal R, Jiang W, Ghetti B. Hallinan GI, et al. Acta Neuropathol. 2021 Aug;142(2):227-241. doi: 10.1007/s00401-021-02336-w. Epub 2021 Jun 14. Acta Neuropathol. 2021. PMID: 34128081 Free PMC article. - In vitro generation of tau aggregates conformationally distinct from parent tau seeds of Alzheimer's brain.
Nam WH, Choi YP. Nam WH, et al. Prion. 2019 Jan;13(1):1-12. doi: 10.1080/19336896.2018.1545524. Epub 2018 Nov 14. Prion. 2019. PMID: 30422056 Free PMC article.
References
- Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K, Iritani S, Tsuchiya K, Iseki E, Yagishita S, Oda T, Mochizuki A. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;1:72–79. doi: 10.1002/ana.10793. - DOI - PubMed
- de Silva R, Lashley T, Gibb G, Hanger D, Hope A, Reid A, Bandopadhyay R, Utton M, Strand C, Jowett T, Khan N, Anderton B, Wood N, Holton J, Revesz T, Lees A. Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol. 2003;1:288–302. doi: 10.1046/j.1365-2990.2003.00463.x. - DOI - PubMed
- de Silva R, Lashley T, Strand C, Shiarli AM, Shi J, Tian J, Bailey KL, Davies P, Bigio EH, Arima K, Iseki E, Murayama S, Kretzschmar H, Neumann M, Lippa C, Halliday G, MacKenzie J, Ravid R, Dickson D, Wszolek Z, Iwatsubo T, Pickering-Brown SM, Holton J, Lees A, Revesz T, Mann DM. An immunohistochemical study of cases of sporadic and inherited frontotemporal lobar degeneration using 3R- and 4R-specific tau monoclonal antibodies. Acta Neuropathol. 2006;1:329–340. doi: 10.1007/s00401-006-0048-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous