Chromatin modifications during repair of environmental exposure-induced DNA damage: a potential mechanism for stable epigenetic alterations - PubMed (original) (raw)
Review
Chromatin modifications during repair of environmental exposure-induced DNA damage: a potential mechanism for stable epigenetic alterations
Heather M O'Hagan. Environ Mol Mutagen. 2014 Apr.
Abstract
Exposures to environmental toxicants and toxins cause epigenetic changes that likely play a role in the development of diseases associated with exposure. The mechanism behind these exposure-induced epigenetic changes is currently unknown. One commonality between most environmental exposures is that they cause DNA damage either directly or through causing an increase in reactive oxygen species, which can damage DNA. Like transcription, DNA damage repair must occur in the context of chromatin requiring both histone modifications and ATP-dependent chromatin remodeling. These chromatin changes aid in DNA damage accessibility and signaling. Several proteins and complexes involved in epigenetic silencing during both development and cancer have been found to be localized to sites of DNA damage. The chromatin-based response to DNA damage is considered a transient event, with chromatin being restored to normal as DNA damage repair is completed. However, in individuals chronically exposed to environmental toxicants or with chronic inflammatory disease, repeated DNA damage-induced chromatin rearrangement may ultimately lead to permanent epigenetic alterations. Understanding the mechanism behind exposure-induced epigenetic changes will allow us to develop strategies to prevent or reverse these changes. This review focuses on epigenetic changes and DNA damage induced by environmental exposures, the chromatin changes that occur around sites of DNA damage, and how these transient chromatin changes may lead to heritable epigenetic alterations at sites of chronic exposure.
Keywords: DNA methylation; histone modifications; reactive oxygen species; toxicants.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
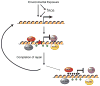
Figure 1. Model for how environmental exposure-induced DNA damage may lead to epigenetic silencing
At sites of environmental exposure, either the exposure itself or increased levels of ROS caused by exposure cause DNA damage (red star). At the sites of damage histone modifications occur (green circles) and the chromatin is remodeled. Additionally, epigenetic silencing proteins are recruited to the site of damage, possibly to prevent transcription from interfering with the repair process. It is unclear at this time if all of these proteins are being recruited to all sites of damage and whether or not they are interacting with each other. In most instances, after completion of DNA repair the chromatin is restored back to normal, the epigenetic silencing proteins are no longer localized to the area, and transcription resumes. In cases of chronic exposure, DNA damage and repair cycles can happen repeatedly and, in rare instances, the chromatin structure may not be returned to normal and binding of the epigenetic silencing proteins may persist, resulting in stable epigenetic silencing.
Similar articles
- The emerging role of epigenetic modifiers in repair of DNA damage associated with chronic inflammatory diseases.
Ding N, Maiuri AR, O'Hagan HM. Ding N, et al. Mutat Res Rev Mutat Res. 2019 Apr-Jun;780:69-81. doi: 10.1016/j.mrrev.2017.09.005. Epub 2017 Sep 28. Mutat Res Rev Mutat Res. 2019. PMID: 31395351 Free PMC article. Review. - An insight into understanding the coupling between homologous recombination mediated DNA repair and chromatin remodeling mechanisms in plant genome: an update.
Banerjee S, Roy S. Banerjee S, et al. Cell Cycle. 2021 Sep;20(18):1760-1784. doi: 10.1080/15384101.2021.1966584. Epub 2021 Aug 26. Cell Cycle. 2021. PMID: 34437813 Free PMC article. Review. - Histone variants in environmental-stress-induced DNA damage repair.
Chen D, Jin C. Chen D, et al. Mutat Res Rev Mutat Res. 2019 Apr-Jun;780:55-60. doi: 10.1016/j.mrrev.2017.11.002. Epub 2017 Nov 21. Mutat Res Rev Mutat Res. 2019. PMID: 31395349 Free PMC article. Review. - Epigenomics in stress tolerance of plants under the climate change.
Kumar M, Rani K. Kumar M, et al. Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review. - Chromatin Remodeling and Epigenetic Regulation in Plant DNA Damage Repair.
Kim JH. Kim JH. Int J Mol Sci. 2019 Aug 22;20(17):4093. doi: 10.3390/ijms20174093. Int J Mol Sci. 2019. PMID: 31443358 Free PMC article. Review.
Cited by
- Acetylation of hMOF Modulates H4K16ac to Regulate DNA Repair Genes in Response to Oxidative Stress.
Zhong J, Ji L, Chen H, Li X, Zhang J, Wang X, Wu W, Xu Y, Huang F, Cai W, Sun ZS. Zhong J, et al. Int J Biol Sci. 2017 Jul 15;13(7):923-934. doi: 10.7150/ijbs.17260. eCollection 2017. Int J Biol Sci. 2017. PMID: 28808424 Free PMC article. - Nucleosome remodelling, DNA repair and transcriptional regulation build negative feedback loops in cancer and cellular ageing.
Watanabe R, Kanno SI, Mohammadi Roushandeh A, Ui A, Yasui A. Watanabe R, et al. Philos Trans R Soc Lond B Biol Sci. 2017 Oct 5;372(1731):20160473. doi: 10.1098/rstb.2016.0473. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28847829 Free PMC article. Review. - Influence of the sperm DNA fragmentation index on the outcome of rescue ICSI and the clinical value of rescue ICSI.
Chen Q, Li D, Cheng J, Xue L, Li J. Chen Q, et al. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022 Jan 28;47(1):63-71. doi: 10.11817/j.issn.1672-7347.2022.210021. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35545364 Free PMC article. Chinese, English. - Deciphering the potential role of post-translational modifications of histones in gastrointestinal cancers: a proteomics-based review with therapeutic challenges and opportunities.
Farrokhi Yekta R, Farahani M, Koushki M, Amiri-Dashatan N. Farrokhi Yekta R, et al. Front Oncol. 2024 Oct 21;14:1481426. doi: 10.3389/fonc.2024.1481426. eCollection 2024. Front Oncol. 2024. PMID: 39497715 Free PMC article. Review. - Epigenetic reduction of DNA repair in progression to gastrointestinal cancer.
Bernstein C, Bernstein H. Bernstein C, et al. World J Gastrointest Oncol. 2015 May 15;7(5):30-46. doi: 10.4251/wjgo.v7.i5.30. World J Gastrointest Oncol. 2015. PMID: 25987950 Free PMC article.
References
- Alegria-Torres JA, Barretta F, Batres-Esquivel LE, Carrizales-Yanez L, Perez-Maldonado IN, Baccarelli A, Bertazzi PA. Epigenetic markers of exposure to polycyclic aromatic hydrocarbons in Mexican brickmakers: a pilot study. Chemosphere. 2013;91:475–480. - PubMed
- Aloia L, Di Stefano B, Di Croce L. Polycomb complexes in stem cells and embryonic development. Development. 2013;140:2525–2534. - PubMed
- Aypar U, Morgan WF, Baulch JE. Radiation-induced epigenetic alterations after low and high LET irradiations. Mutat Res. 2011;707:24–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources