VEGF targets the tumour cell - PubMed (original) (raw)
Review
VEGF targets the tumour cell
Hira Lal Goel et al. Nat Rev Cancer. 2013 Dec.
Abstract
The function of vascular endothelial growth factor (VEGF) in cancer is not limited to angiogenesis and vascular permeability. VEGF-mediated signalling occurs in tumour cells, and this signalling contributes to key aspects of tumorigenesis, including the function of cancer stem cells and tumour initiation. In addition to VEGF receptor tyrosine kinases, the neuropilins are crucial for mediating the effects of VEGF on tumour cells, primarily because of their ability to regulate the function and the trafficking of growth factor receptors and integrins. This has important implications for our understanding of tumour biology and for the development of more effective therapeutic approaches.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures
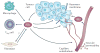
Figure 1. VEGF functions in tumours
Vascular endothelial growth factor (VEGF) that is secreted by tumour and stromal cells, including macrophages, endothelial cells and fibroblasts, has multiple functions in the tumour microenvironment, which involve the ability of VEGF to interact with VEGF receptors that are expressed on different cell types. VEGF functions as a primary stimulus for angiogenesis, which is a process that involves the ability of VEGF receptors to stimulate signalling pathways that induce the proliferation and the migration of endothelial cells, and the ability of these cells to degrade and to remodel the extracellular matrix. These processes culminate in sprouting angiogenesis and the formation of new blood vessels. VEGF can also increase vascular permeability, which results in the deposition of a provisional fibrin matrix that triggers the formation of desmoplastic stroma. By contrast, VEGF secreted by tumour cells functions in an autocrine manner and promotes dedifferentiation and an epithelial–mesenchymal transition phenotype, with a consequent enhancement of tumour invasion and survival, and it can facilitate the function of cancer stem cells (FIG. 3). VEGF can also function as a chemoattractant to recruit regulatory T (TReg) cells that inhibit an antitumour immune response. Tumour fibroblasts also secrete VEGF. Neuropilin 1 that is expressed on tumour fibroblasts may contribute to tumour growth by nucleating fibronectin fibril formation, but it is not known whether this process involves VEGF. Arrows indicate the source and the targets of VEGF in tumours.
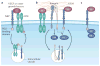
Figure 2. Receptor interactions that promote VEGF signalling in tumour cells, and the central role of NRPs
a | Neuropilins (NRPs) interact with and potentiate the signalling function of growth factor receptor tyrosine kinases (RTKs), including vascular endothelial growth factor (VEGF) RTKs. This mode of regulation may be associated with internalization of the RTK and signalling from an intracellular compartment. Several growth factors, including hepatocyte growth factor, basic fibroblast growth factor, platelet-derived growth factor and transforming growth factor-β, directly interact with NRPs, but whether this binding is sufficient by itself to induce a signalling response is not known. b | NRPs also interact with specific integrins and activate their ability to bind to extracellular matrix (ECM) ligands, which results in the stimulation of integrin-mediated signalling through focal adhesion kinase (FAK). The RTK VEGF receptor 2 (VEGFR2) can also function in a similar capacity. In addition, NRPs may regulate integrin function by promoting their endocytic recycling. Both NRPs and specific integrin α-subunits (α5 and α6) contain a PDZ (PSD95, DLG and ZO1)-binding domain (Ser-Glu-Ala) at their carboxyl terminus, and PDZ proteins, such as the neuropilin-interacting protein GIPC1 might promote the association of these two classes of receptors. c | NRPs may also signal independently of other receptors, possibly by using their PDZ-binding domains to associate with signalling molecules such as ABL1. Note that these proposed mechanisms are not mutually exclusive, and there is the possibility that VEGF signalling in tumour cells involves the formation of macromolecular complexes that integrate components of these mechanisms.
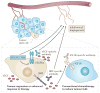
Figure 3. Role of autocrine VEGF signalling in the function of cancer stem cells and tumour formation
a | The expression of vascular endothelial growth factor (VEGF) and VEGF receptors is induced concomitantly with oncogenic transformation; this facilitates the establishment of autocrine VEGF signalling. This signalling, which is mediated by the receptor tyrosine kinase VEGF receptor 2 (VEGFR2) and by neuropilins (NRPs), could be necessary for the function of cancer stem cells (beige cells) because it seems to maintain the size of the stem cell pool and to sustain self-renewal. The ability of autocrine VEGF signalling that is mediated by NRPs and integrins to regulate the expression of the Hedgehog target GLI family zinc finger 1 (GLI1) and the Polycomb group repressor BMI1 provides one mechanism to account for the contribution of autocrine VEGF signalling to the function of cancer stem cells, but other mechanisms probably exist. b | Cancer stem cells can be localized in a perivascular niche, which enables VEGF that is secreted by these cells to function in a paracrine manner to stimulate angiogenesis in nascent tumours. Autocrine VEGF signalling can also promote dedifferentiation and an epithelial–mesenchymal transition (EMT) phenotype that results in increased migration and invasion into the stroma. FAK, focal adhesion kinase.
Figure 4. Therapeutic targeting of VEGF signalling in tumour cells
The functional importance of vascular endothelial growth factors (VEGFs) and VEGF receptors — that is, neuropilins (NRPs) and VEGF receptor tyrosine kinases (RTKs) — that are expressed by tumour cells, in particular those that are expressed by cancer stem cells (beige cells), provides an important opportunity for the development of new therapeutic approaches, especially for highly aggressive tumours. These approaches have the potential to promote tumour regression and to improve the response to standard chemotherapy and radiation therapy. So far, strategies that inhibit VEGF signalling have primarily focused on targeting angiogenesis using either bevacizumab to inhibit VEGF or tyrosine kinase inhibitors (TKIs) that target VEGF RTKs such as VEGF receptor 2 (VEGFR2). NRPs are becoming recognized as crucial effectors of autocrine VEGF signalling in tumours, and more emphasis should be placed on targeting them therapeutically. Although some side effects were observed during the initial clinical use of a humanized NRP1 antibody, targeting NRPs is still a potentially effective strategy, and approaches need to be developed that minimize these side effects. It is also important to note that bevacizumab does not inhibit the binding of VEGF to NRPs, which highlights the importance of targeting NRPs directly and developing VEGF-specific reagents (such as placental growth factor (PLGF)-specific antibodies) that inhibit NRP binding. Targeting NRPs can result in compensatory signalling by other growth factor receptors, which indicates the potential importance of using a combination therapy. This possibility is shown by the compensatory insulin-like growth factor 1 receptor (IGF1R) signalling that occurs in prostate cancer, and it is likely that other mechanisms of compensation in response to VEGF pathway inhibition will be discovered for other tumour cells. These approaches may benefit from the use of conventional chemotherapy to reduce overall tumour burden.
Similar articles
- VEGF/Neuropilin Signaling in Cancer Stem Cells.
Mercurio AM. Mercurio AM. Int J Mol Sci. 2019 Jan 23;20(3):490. doi: 10.3390/ijms20030490. Int J Mol Sci. 2019. PMID: 30678134 Free PMC article. Review. - Signal transduction by vascular endothelial growth factor receptors.
Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Koch S, et al. Biochem J. 2011 Jul 15;437(2):169-83. doi: 10.1042/BJ20110301. Biochem J. 2011. PMID: 21711246 Review. - Neuropilin signalling in angiogenesis.
Koch S. Koch S. Biochem Soc Trans. 2012 Feb;40(1):20-5. doi: 10.1042/BST20110689. Biochem Soc Trans. 2012. PMID: 22260660 Review. - Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF).
Byrne AM, Bouchier-Hayes DJ, Harmey JH. Byrne AM, et al. J Cell Mol Med. 2005 Oct-Dec;9(4):777-94. doi: 10.1111/j.1582-4934.2005.tb00379.x. J Cell Mol Med. 2005. PMID: 16364190 Free PMC article. Review. - Vascular endothelial growth factor receptors: expression and function in solid tumors.
Wey JS, Stoeltzing O, Ellis LM. Wey JS, et al. Clin Adv Hematol Oncol. 2004 Jan;2(1):37-45. Clin Adv Hematol Oncol. 2004. PMID: 16163158 Review.
Cited by
- Moss-made pharmaceuticals: from bench to bedside.
Reski R, Parsons J, Decker EL. Reski R, et al. Plant Biotechnol J. 2015 Oct;13(8):1191-8. doi: 10.1111/pbi.12401. Epub 2015 May 25. Plant Biotechnol J. 2015. PMID: 26011014 Free PMC article. Review. - MicroRNA-144: A novel biological marker and potential therapeutic target in human solid cancers.
Zhou M, Wu Y, Li H, Zha X. Zhou M, et al. J Cancer. 2020 Sep 25;11(22):6716-6726. doi: 10.7150/jca.46293. eCollection 2020. J Cancer. 2020. PMID: 33046994 Free PMC article. Review. - Prospects of circular RNAs: the regulators of drug resistance and metastasis in gastric cancer.
Wang B, Chen Z, Liu W, Tan B. Wang B, et al. Am J Transl Res. 2022 Aug 15;14(8):5760-5772. eCollection 2022. Am J Transl Res. 2022. PMID: 36105039 Free PMC article. Review. - FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review).
Katoh M. Katoh M. Int J Mol Med. 2016 Jul;38(1):3-15. doi: 10.3892/ijmm.2016.2620. Epub 2016 May 31. Int J Mol Med. 2016. PMID: 27245147 Free PMC article. Review. - A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells.
Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, Ingerpuu S, Patarroyo M, Cao S, Lim E, Mao J, McKee KK, Yurchenco PD, Mercurio AM. Chang C, et al. Genes Dev. 2015 Jan 1;29(1):1-6. doi: 10.1101/gad.253682.114. Genes Dev. 2015. PMID: 25561492 Free PMC article.
References
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. - PubMed
- Tischer E, et al. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun. 1989;165:1198–1206. - PubMed
- Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. - PubMed
- Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources