Adaptation of the Haloarcula hispanica CRISPR-Cas system to a purified virus strictly requires a priming process - PubMed (original) (raw)
Adaptation of the Haloarcula hispanica CRISPR-Cas system to a purified virus strictly requires a priming process
Ming Li et al. Nucleic Acids Res. 2014 Feb.
Abstract
The clustered regularly interspaced short palindromic repeat (CRISPR)-Cas system mediates adaptive immunity against foreign nucleic acids in prokaryotes. However, efficient adaptation of a native CRISPR to purified viruses has only been observed for the type II-A system from a Streptococcus thermophilus industry strain, and rarely reported for laboratory strains. Here, we provide a second native system showing efficient adaptation. Infected by a newly isolated virus HHPV-2, Haloarcula hispanica type I-B CRISPR system acquired spacers discriminatively from viral sequences. Unexpectedly, in addition to Cas1, Cas2 and Cas4, this process also requires Cas3 and at least partial Cascade proteins, which are involved in interference and/or CRISPR RNA maturation. Intriguingly, a preexisting spacer partially matching a viral sequence is also required, and spacer acquisition from upstream and downstream sequences of its target sequence (i.e. priming protospacer) shows different strand bias. These evidences strongly indicate that adaptation in this system strictly requires a priming process. This requirement, if validated also true for other CRISPR systems as implied by our bioinformatic analysis, may help to explain failures to observe efficient adaptation to purified viruses in many laboratory strains, and the discrimination mechanism at the adaptation level that has confused scientists for years.
Figures
Figure 1.
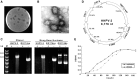
Isolation and characterization of a new halovirus HHPV-2. (A) HHPV-2 plaques on the H. hispanica lawn. (B) A representative transmission electron micrograph of negative-stained HHPV-2 virions. Bar, 50 nm. (C) DNase I (left) and Mung Bean Nuclease (right) digestions of the HHPV-2 genome, which proved to be an ssDNA molecule. The ssDNA genome (ФX174ss) and replicative form (ФX174ds) of phiX174 were also subjected to digestions. Lane M, dsDNA size marker. (D) A schematic depiction of the HHPV-2 genome. Similarities between HHPV-1 [a closely related dsDNA virus (23)] and HHPV-2 homologous gene products are shown. (E) Growth curve of uninfected (solid line) and virus-infected (dotted line) H. hispanica DF60 cultures.
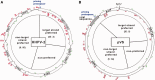
Figure 2.
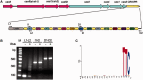
CRISPR adaptation to HHPV-2 infection. (A) Depiction of the single CRISPR structure and the preceding cas operon carried by the H. hispanica ATCC 33960 genome. Primers used to examine CRISPR expansion (in panel B) are shown as black arrows and listed in
Supplementary Table S2
. (B) PCR assay to detect CRISPR expansion at the leader end (L1–L2), the inner part (I1–I2) or the distal end (D1–D2). DNA sampled from infected (+) or uninfected (−) cells was used as PCR templates. Lane M, dsDNA size marker. (C) The sequence logo showing the conserved PAM of TTC. The 20 nt upstream of each protospacer observed during HHPV-2 infection were collected and analyzed with WebLogo (
http://weblogo.berkeley.edu/logo.cgi
).
Figure 3.
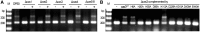
Adaptation to HHPV-2 infection under different cas genetic backgrounds. (A) Cas requirement for adaptation. For each cas mutant, DNA was sampled from cells transformed with an empty plasmid (−) or the plasmid carrying the deleted cas gene(s) (+). The plasmid-carried cas gene(s) was/were under the control of the cas operon promoter. (B) Requirements for the nuclease and helicase activities of Cas3. Alanine replacement was performed for the putative key residues in the HD nuclease domain (H20A, H55A, D56A and D229A) and the DExD/H helicase domain (K315A, D439A and E440A). Another two conserved residues (His6 and Lys113) were also mutated. The empty plasmid (−) and the plasmid carrying a wild-type Cas3 (Cas3WT) were used, respectively, as negative and positive controls. Lane Ms, dsDNA size markers.
Figure 4.
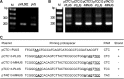
A preexisting spacer (spacer13) matching the HHPV-2 genome was required. (A) Depiction of the imperfect match between spacer13 and an HHPV-2 fragment. Positions of the fragment on the HHPV-2 genome are indicated under the sequence. Three upstream nucleotides corresponding to PAM motif are underlined. (B) Depiction of the spacer content of CRISPR variants. Δsp2–6, Δsp7–13, Δsp2–12, Δsp2–13 and Δsp1–14 are designated according to the spacers (or repeat) bordering the omitted region (dashed lines). In Δsp13, spacer13 and the immediately upstream repeat were deleted. (C) Adaptation of variant CRISPRs to HHPV-2 infection. Lane M, dsDNA size marker.
Figure 5.
Adaptation to engineered plasmids. (A) Adaptation to a modified pWL502 plasmid (pVS) carrying a viral sequence that is partially matched by spacer13. The empty plasmid (pWL502) was used as a control. (B) Adaptation to modified pWL502 plasmids carrying different artificial priming protospacers. (C) Information of the engineered plasmids in panel B. The plasmid designations indicate their PAM sequences (CTC, TCC or TAC) and different priming protospacers (partially matched by spacer1 or spacer13) on different strands (+ or −). Within the priming protospacer sequence, nucleotides that were designed mismatching the corresponding spacer are shown in bold and underlined. The plus (+) and minus (−) strands correspond, respectively, to the coding and template strands of the pyrF gene. These engineered plasmids were transformed into wild-type DF60 cells and examined for CRISPR expansion. Land Ms, dsDNA size markers.
Figure 6.
Spacer acquisition shows a strand bias directed by the priming spacer. The priming protospacer, which is imperfectly targeted by the priming spacer (spacer13), is shown as a blue arrow. Protospacers from which new spacers were acquired during HHPV-2 infection (A) and pVS transformation (B) are shown as red or green arrows. Red arrows indicate protospacers located on the preferred strand, whereas the green ones indicate those on the non-preferred strand or within the non-preferred genomic region. For each region, the ratio of protospacers on the two strands (target versus non-target) is given. For HHPV-2, the double-stranded replication form genome is shown. The plasmid pVS carries an HHPV-2 fragment (shadowed) containing the priming protospacer. The crRNA of spacer13 is expected to base pair to the target strand of the priming protospacer (the template strand of the rep or pyrF gene) and displace the non-target strand (the coding strand of the rep or pyrF gene).
Figure 7.
Imperfect matches between preexisting spacers and viral (or phage) DNA. (A) S. thermophilus DGCC7710 CRISPR1 (under the GenBank accession number EF434469) contains preexisting spacers matching the Ф858 and Ф2972 genomes. (B) Representative matches between haloarchaeal spacers and haloviral genomes. The CRISPR IDs used in the CRISPRdb database (
http://crispr.u-psud.fr/crispr/
) are given. The match between H. hispanica spacer13 and HHPV-2 is also shown as a reference. The _E_-value of each match is shown. Dots in the viral (or phage) sequences indicate nucleotides identical to spacers at these positions. The genome positions of the viral (or phage) sequences are indicated by two numbers separated by a colon.
Figure 8.
A possible priming model explaining the strand bias changes between the DNA sequences upstream and downstream of the priming protospacer. Despite several mismatches between the crRNA of the priming spacer and the target DNA, Cascade still binds to the latter with a reduced affinity, forming the R-loop. Cas3 is then recruited to the non-target strand and nicks it. In a possible Cascade replacement process, H. hispanica Cas3 may occasionally flip onto the target strand via an unknown mechanism. The Cas3 helicase (or translocase) activity may facilitate the 3′–5′ movement of the adaptation machinery on the upstream non-target strand or on the downstream target strand of the priming protospacer.
Similar articles
- Haloarcula hispanica CRISPR authenticates PAM of a target sequence to prime discriminative adaptation.
Li M, Wang R, Xiang H. Li M, et al. Nucleic Acids Res. 2014 Jun;42(11):7226-35. doi: 10.1093/nar/gku389. Epub 2014 May 6. Nucleic Acids Res. 2014. PMID: 24803673 Free PMC article. - Cas4 Nucleases Can Effect Specific Integration of CRISPR Spacers.
Zhang Z, Pan S, Liu T, Li Y, Peng N. Zhang Z, et al. J Bacteriol. 2019 May 22;201(12):e00747-18. doi: 10.1128/JB.00747-18. Print 2019 Jun 15. J Bacteriol. 2019. PMID: 30936372 Free PMC article. - Protospacer-Adjacent Motif Specificity during Clostridioides difficile Type I-B CRISPR-Cas Interference and Adaptation.
Maikova A, Boudry P, Shiriaeva A, Vasileva A, Boutserin A, Medvedeva S, Semenova E, Severinov K, Soutourina O. Maikova A, et al. mBio. 2021 Aug 31;12(4):e0213621. doi: 10.1128/mBio.02136-21. Epub 2021 Aug 24. mBio. 2021. PMID: 34425703 Free PMC article. - A short overview of the CRISPR-Cas adaptation stage.
Mosterd C, Rousseau GM, Moineau S. Mosterd C, et al. Can J Microbiol. 2021 Jan;67(1):1-12. doi: 10.1139/cjm-2020-0212. Epub 2020 Jun 19. Can J Microbiol. 2021. PMID: 32559396 Review. - Creating memories: molecular mechanisms of CRISPR adaptation.
Lee H, Sashital DG. Lee H, et al. Trends Biochem Sci. 2022 Jun;47(6):464-476. doi: 10.1016/j.tibs.2022.02.004. Epub 2022 Feb 28. Trends Biochem Sci. 2022. PMID: 35236570 Free PMC article. Review.
Cited by
- Two distinct DNA binding modes guide dual roles of a CRISPR-Cas protein complex.
Blosser TR, Loeff L, Westra ER, Vlot M, Künne T, Sobota M, Dekker C, Brouns SJJ, Joo C. Blosser TR, et al. Mol Cell. 2015 Apr 2;58(1):60-70. doi: 10.1016/j.molcel.2015.01.028. Epub 2015 Mar 5. Mol Cell. 2015. PMID: 25752578 Free PMC article. - An archaeal CRISPR type III-B system exhibiting distinctive RNA targeting features and mediating dual RNA and DNA interference.
Peng W, Feng M, Feng X, Liang YX, She Q. Peng W, et al. Nucleic Acids Res. 2015 Jan;43(1):406-17. doi: 10.1093/nar/gku1302. Epub 2014 Dec 10. Nucleic Acids Res. 2015. PMID: 25505143 Free PMC article. - Foreign DNA acquisition by the I-F CRISPR-Cas system requires all components of the interference machinery.
Vorontsova D, Datsenko KA, Medvedeva S, Bondy-Denomy J, Savitskaya EE, Pougach K, Logacheva M, Wiedenheft B, Davidson AR, Severinov K, Semenova E. Vorontsova D, et al. Nucleic Acids Res. 2015 Dec 15;43(22):10848-60. doi: 10.1093/nar/gkv1261. Epub 2015 Nov 19. Nucleic Acids Res. 2015. PMID: 26586803 Free PMC article. - Conserved DNA motifs in the type II-A CRISPR leader region.
Van Orden MJ, Klein P, Babu K, Najar FZ, Rajan R. Van Orden MJ, et al. PeerJ. 2017 Apr 4;5:e3161. doi: 10.7717/peerj.3161. eCollection 2017. PeerJ. 2017. PMID: 28392985 Free PMC article. - Programming Native CRISPR Arrays for the Generation of Targeted Immunity.
Hynes AP, Labrie SJ, Moineau S. Hynes AP, et al. mBio. 2016 May 3;7(3):e00202-16. doi: 10.1128/mBio.00202-16. mBio. 2016. PMID: 27143383 Free PMC article.
References
- Sorek R, Kunin V, Hugenholtz P. CRISPR–a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat. Rev. Microbiol. 2008;6:181–186. - PubMed
- van der Oost J, Jore MM, Westra ER, Lundgren M, Brouns SJ. CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem. Sci. 2009;34:401–407. - PubMed
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. - PubMed
- Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 2005;151:653–663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources