Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways - PubMed (original) (raw)
. 2013 Dec 19;504(7480):394-400.
doi: 10.1038/nature12776. Epub 2013 Nov 24.
Laura E Clarke # 1, Gordon X Wang # 2, Benjamin K Stafford 3, Alexander Sher 4, Chandrani Chakraborty 1, Julia Joung 1, Lynette C Foo 5, Andrew Thompson 6, Chinfei Chen 6, Stephen J Smith 2, Ben A Barres 1
Affiliations
- PMID: 24270812
- PMCID: PMC3969024
- DOI: 10.1038/nature12776
Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways
Won-Suk Chung et al. Nature. 2013.
Abstract
To achieve its precise neural connectivity, the developing mammalian nervous system undergoes extensive activity-dependent synapse remodelling. Recently, microglial cells have been shown to be responsible for a portion of synaptic pruning, but the remaining mechanisms remain unknown. Here we report a new role for astrocytes in actively engulfing central nervous system synapses. This process helps to mediate synapse elimination, requires the MEGF10 and MERTK phagocytic pathways, and is strongly dependent on neuronal activity. Developing mice deficient in both astrocyte pathways fail to refine their retinogeniculate connections normally and retain excess functional synapses. Finally, we show that in the adult mouse brain, astrocytes continuously engulf both excitatory and inhibitory synapses. These studies reveal a novel role for astrocytes in mediating synapse elimination in the developing and adult brain, identify MEGF10 and MERTK as critical proteins in the synapse remodelling underlying neural circuit refinement, and have important implications for understanding learning and memory as well as neurological disease processes.
Figures
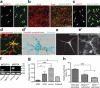
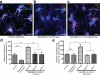
Figure 1. Localization of MEGF10 and MERTK to astrocytes and their phagocytic roles in purified astrocytes
a, Confocal images showing specific MEGF10 (red) localization to astrocytes (green, arrows). b, β-gal (red) expression driven by endogenous Megf10 locus in astrocytes (green). c, Confocal images showing MERTK (red) localization to astrocytes (green, arrows) as well as microglia (arrowheads) and endothelial cells (asterisks). d-d’, 3D surface rendering before (d) after (d’) subtracting synaptosomes (red) outside of the astrocytic volume (cyan). e-e’, pHrodo-conjugated synaptosomes (e’) engulfed by astrocytes (e). f, Western blotting showing the absence of functional MEGF10 and MERTK proteins in the P5 Megf10—/— and Mertk—/— brains, respectively. g, Compared to AGM, addition of ACM, 5% serum or Protein S significantly increased the PI of astrocytes. h, Purified Megf10—/— and Mertk—/— astrocytes showed a 42 and 64 % reduction in the engulfment ability, respectively. Representative data from three independent experiments (g, h). P value: *P<0.05, ***P<0.001, One-way ANOVA. error bars, s.e.m. Scale bar, 20 μm (a,c,d,e’), 100 μm (b) and 10 μm (d’).
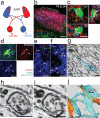
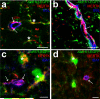
Figure 2. Astrocytes mediate synapse elimination in the developing dLGN
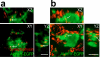
a, Schematic diagram of anterograde RGC labeling with CTB-594 (red) and CTB-647 (blue), projecting to the developing dLGN. b, Contra- (red) and ipsi- (blue) lateral projections in the P6 dLGN from _Aldh1l1_-EGFP (green) transgenic animals. Scale bar, 100 μm. c, Confocal optical sections through XY, XZ and YZ axis showing CTB-labeled debris (arrows) inside of astrocytes (green). Scale bar, 10 μm. d, 3D-max projection showing that CTB-labeled debris (red, arrows) engulfed by astrocytes (green) were co-localized with LAMP2-positive lysosomes (blue). Scale bar, 10 μm. e-f, 3D-max projection AT images showing engulfed putative synapses (arrows) in astrocytes (blue). Engulfed putative synapses are defined as EYFP (blue) enveloped CTB-594 (magenta) and PSD-95 (e) or GluR1 (f) (green) that are within 200nm of each other. Scale bar, 5 μm. g, Low magnification SBEM micrograph showing an intact synapse (yellow rectangle, note the presynaptic vesicles and postsynaptic density) and astrocytic process (cyan) containing engulfed presynaptic material (red rectangle, note the vesicles that are the same size as the presynaptic vesicles). h-i, Consecutive high magnification SBEM micrographs showing the engulfed presynaptic structure with irregular double-membrane morphology (arrows) and second inclusion (asterisks). j, 3D-reconstruction of SBEM micrographs confirmed astrocytic processes (cyan) with glycogen granules (arrows) fully engulfed presynaptic material (red arrowhead) as well as the second inclusion (black arrowhead). Synaptic vesicles in the intact synapse (yellow rectangles) in the axons (orange) and the engulfed presynaptic material were shown in red and yellow spheres, respectively. Scale bar, 0.4 μm (g, h, i).
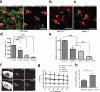
Figure 3. Astrocytes mediate developmental synapse pruning and remodeling through MEGF10 and MERTK pathways
a, Confocal images of the wild type P6 dLGN before and after subtracting CTB-594 (pseudo-colored green) outside of astrocytes (pseudo-colored red). bc, In Megf10—/— (b) and Mertk—/— (c) mice, the amount of engulfed CTB-594 (green) by astrocytes (red) was significantly reduced (arrows). d, The highest astrocyte-mediated phagocytosis occurs during P4-P6. n = 3 mice/age. e, Megf10—/— and Mertk—/— mice showed significantly reduced astrocyte-mediated phagocytosis (45 and 58 % reduction, respectively), which was further reduced in Megf10—/—; Mertk—/— mice (85 % reduction). n = 4 mice/group. f-g, Overlap between contra- and ipsi-lateral projections was significantly increased in Megf10—/—; Mertk—/— mice at P30 (f), independently from the threshold value used in the analysis (g). n = 4 mice/group. h, The area of dLGN occupied by ipsi-lateral projections was significantly increased in P30 Megf10—/—; Mertk—/— mice. n = 4 mice/group. P value: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, One-way ANOVA for all except h (_t_-test). NS, not significant. error bars, s.e.m. Scale bar, 20 μm (a,b,c) and 100 μm (f).
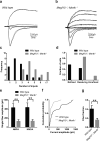
Figure 4. dLGN neurons are abnormally innervated by multiple weak inputs in Megf10—/—; Mertk—/— mice
a-b, Representative traces of superimposed EPSCs evoked by increasing intensities of optic tract stimulation recorded from individual wild type (a) and Megf10—/—; Mertk—/— (b) dLGN neurons at P15-18. c, Megf10—/—; Mertk—/— neurons receive a significantly larger number of inputs (p=0.003). d, The majority of the Megf10—/—; Mertk—/— neurons were in the resolving or unrefined category whereas most of wild type neurons were in the refined category. e, Megf10—/—; Mertk—/— neurons had significantly smaller AMPA (p=0.002) and NMDA (p=0.008) single fiber currents. f, Megf10—/—; Mertk—/— neurons had significantly more small amplitude single fiber AMPA current responses in cumulative probability histograms (p=0.023). g, Megf10—/—; Mertk—/— neurons had a significantly reduced mean fiber fraction (FF) (p=0.005). Recordings were obtained from 23 wild type cells and 20 Megf10—/—; Mertk—/— cells. P value: **P<0.01, _t_-test except c and f (Kolmogorov-Smirnov test). error bars, s.e.m
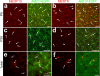
Figure 5. Neural activity promotes astrocyte-mediated synapse elimination through MEGF10 and MERTK
a-c, 3D-max projection confocal images showing CTB-594-labeled contra-lateral (red) and CTB-647-labeled ipsi-lateral (green) debris engulfed by astrocytes (blue) in P6 dLGN, comparing control (a), binocular (b) and monocular (c) injections. Red and green debris co-localized in astrocytes (blue) appeared as bright yellow. d, Relative engulfment of contra-lateral projections by astrocytes was significantly reduced after binocular epibatidine injections whereas it was significantly increased after monocular epibatidine injections to the wide type contra-lateral eye. However, this increased engulfment was reduced in Megf10—/— or Mertk—/— background. e, In binocular epibatidine injection cases, the ratio of engulfed contra- vs. ipsi-lateral projections by astrocytes was unchanged compared to control injections whereas it was significantly increased after monocular epibatidine injections to wild type contra-lateral eye. However, this increased ratio of engulfed contra- vs. ipsi-lateral projections was reduced in Megf10—/— or Mertk—/— background. n = 4 mice/treatment. P value: *P<0.05, **P<0.01, ***P<0.001, One-way ANOVA. NS, not significant. error bars, s.e.m. Scale bar, 10 μm (a-c).
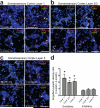
Figure 6. Astrocytes in the adult cortex continuously engulf synapses
a-c, 3D-max projection AT images showing EYFP-labeled cortical astrocytes (blue) and engulfed putative excitatory (Bassoon: magenta and PSD-95: cyan) and inhibitory synapses (VGAP: yellow and Gephysin: red) from layers 1 (a), 2/3 (b) and 5 (c) of the 1- and 4-month old mouse somatosensory cortex (Total volume = 60 μm by 60 μm by 2.8 μm). Scale bar, 2.5 μm. d, The density ratio of engulfed excitatory and inhibitory synapses by astrocytes between the 1-month and 4-month old cortex. n = 3 mice/age. P value: *P<0.05, _t_-test. error bars, s.e.m.
Extended Data Figure 1. MERTK protein is localized to multiple cell types
a-b, Confocal P5 dLGN images showing MERTK (red) protein expression in endothelial cells (arrows) stained with BSL (blue) as well as in astrocytic processes (asterisks) labeled by _Aldh1l1_-EGFP (green). c-d, Confocal P5 dLGN images showing MERTK (red) protein expression in microglia (arrows) stained with IBA1 (blue) as well as in astrocytes (asterisks) labeled by _Aldh1l1_-EGFP (green). Scale bar, 10 μm.
Extended Data Figure 2. MEGF10 and MERTK are continuously localized to cortical astrocytes throughout animal life
MEGF10 (red; a, c, e) and MERTK (red; b, d, f) are localized to cortical astrocytes (arrows) labeled by _Aldh1l1_-EGFP (green) in the P5 (a, b), P30 (c, d) and 1 year old (e, f) mouse cortex. While MEGF10 is specifically localized to astrocytes, MERTK is also localized to microglia (arrowheads) as well as endothelial cells (asterisks). Scale bar, 20 μm
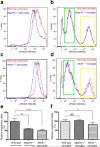
Extended Data Figure 3. Phagocytic capacity of Megf10—/— or Mertk—/— astrocytes and microglia measured by FACS
a-d, FACS profiles of astrocytes (a, c) and enriched microglia population (b, d) for pHrodo intensity after incubating with pHrodo-conjugated synaptosomes for 24 hours in the presence of 5% serum. Megf10—/— (a) and Mertk—/— (c) astrocytes (blue lines) showed clear leftward shifts in pHrodo intensity compared to wild type astrocytes (red lines). Megf10—/— microglia (b, blue line) didn't show any difference in the FACS profile compared to wild type microglia (red lines in b). Mertk—/— microglia (d, blue line) exhibited a slight leftward shift in the FACS profile showing strong pHrodo intensity (yellow rectangle) whereas there was no difference in low pHrodo intensity (green rectangle) compared to wild type microglia. e, Megf10—/— and Mertk—/— astrocytes showed a 42 and 51 % reduction in the relative engulfment ability, respectively, compared to wild type astrocytes. f, Mertk—/— microglia showed a 25% reduction in the relative engulfment ability compared to wild type microglia. The relative engulfment ability was calculated by comparing the percentage of the cell population expressing strong pHrodo intensity (> 3*10^4). Representative data from three independent experiments. P value: *P<0.05, **P<0.01, ***P<0.001, One-way ANOVA. NS, not significant. error bars, s.e.m.
Extended Data Figure 4. Astrocytes in the developing dLGN engulf pre-synaptic material
a-b, Optical sections of the P5 dLGN using structured illumination (a) and confocal (b) microscopy through XY, XZ and YZ axis showed Synaptophysin- (a, arrows) and VGlut2- (b, arrows) positive presynaptic material were engulfed by EGFP-expressing astrocytes (green). Scale bar, 1 μm (a) and 5 μm (b).
Extended Data Figure 5. Astrocytes in the developing dLGN engulf pre- and post-synaptic material, revealed by array tomography (AT)
a, 3D-max projection AT images showing EYFP (grey)-labeled P5 dLGN astrocytes (Total volume = 155 μm by 125 μm by 2.8 μm). b, Close-up view of EYFP (blue)-labeled dLGN astrocytes. c-d, Close-up view of 3D-max projection AT images showing CTB-594 labeled projections (magenta) before (c) and after (d) image processing, revealing engulfed CTB-labeled debris by astrocytes (blue). e-f, Close-up view of 3D-max projection AT images showing Bassoon (red) before (e) and after (f) image processing, revealing engulfed Bassoon-positive synaptic material by astrocytes (blue). g-h, Close-up view of max projection AT images showing PSD-95 (cyan) before (g) and after (h) image processing, revealing engulfed PSD-95-positive synaptic material by astrocytes (blue). i-j, Close-up view of 3D-max projection AT images showing GluR1 (green) before (i) and after (j) image processing, revealing engulfed GluR1-positive synaptic material by astrocytes (blue). Scale bar, 50 μm (a) and 20 μm (b-j).
Extended Data Figure 6. Astrocytes clear neural debris more robustly than microglia in the developing dLGN
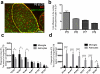
a, Representative image of P6 dLGN (yellow dotted line) showing astrocytes labeled by _Aldh1l1_-EGFP (green) and microglia labeled by IBA1 staining (red). b, The number of astrocytes in dLGN is much greater than microglia at P5 (10 folds), P6 (7 folds), P7 (6 folds) and P9 (4 folds). c, The PI measured by the total amount of CTB debris/ unit cell volume showed that during P3-P6, microglia engulfed more CTB-labeled debris than astrocytes per unit cell volume, whereas astrocytes and microglia cleared about the same amount of debris per unit cell volume after P6. n = 5/group. d, The PI measured by the total amount of CTB debris per imaging field showed that astrocytes clear the significantly greater amount of CTB debris than microglia during P3-P9. n = 5/group. *P<0.05, ***P<0.001, _t_-test. error bars, s.e.m.
Extended Data Figure 7. MERTK is dispensable for the microglia-mediated phagocytosis in developing dLGN
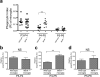
a, Comparing the PI of microglia in dLGN during P3-P6 between wild type and Mertk—/— mice. Microglia showed the gradual decrease in the PI measured from P3 to P6. bd, Relative engulfment ability between wild type and Mertk—/— microglia during P3-P4 (b), P4-P5 (c) and P5-P6 (d). Mertk—/— microglia showed a transient increase in the PI during P4-P5. However, the PI of microglia during P3-P4 and P5-P6 were comparable between wild type and Mertk—/— mice. n = 4/each group. **P<0.01, _t_-test. NS, not significant. error bars, s.e.m.
Extended Data Figure 8. Spontaneous retinal wave is intact in Megf10—/—; Mertk—/— mice
a, Waves occur with the same frequency (left), propagate at the same speed (middle), and are the same size in Megf10—/—; Mertk—/— retinas (right). a’, Correlation index (CI), computed for spike trains from pairs of neurons and plotted as a function of the distance between electrodes on which the neurons were recorded, shows that CI decreases as a function of distance in both wild type and Megf10—/—; Mertk—/— retinas. Plots summarize data from multiple wild type (black; n = 5) and Megf10—/—; Mertk—/— (red; n = 5) preparations. b, Bursts fired by ganglion cells show no difference in duration (top left), mean spike rate (top middle), or the amount of time spent firing at high frequencies in Megf10—/—; Mertk—/— retinas (top right). The percent of all spikes that are incorporated into bursts (bottom left), and the percent of all bursts that occur during waves (bottom right), are also unchanged in Megf10—/—; Mertk—/— retinas. Plots summarize data from multiple wild type (black; n = 5) and Megf10—/—; Mertk—/— (red; n = 5) preparations. c, Quantification of directionality parameters demonstrates that the same fraction of ganglion cells demonstrate a directional bias in Megf10—/—; Mertk—/— retinas (top). In addition, the magnitude of the directional bias of all neurons in a preparation (bottom) is unchanged in Megf10—/—; Mertk—/— retinas. Plots summarize data from multiple wild type (black; n = 5) and Megf10—/—; Mertk—/— (red; n = 5) preparations. _t_-test.
Extended Data Figure 9. Analysis of astrocytic and synaptic protein localization by array tomography (AT) in the adult cortex with EYFP-expressing astrocytes
a, 3D-max projection AT images showing EYFP (grey)-labeled astrocytes from the 4-month old somatosensory cortex (Total volume = 155 μm by 125 μm by 2.8 μm). b, Close-up view of EYFP (grey)-labeled cortical astrocytes. c-f, Close-up views of 3D-max projection AT images showing EAA2 (c-d; green) and Glutamine Synthetase (e-f; magenta) staining reveal specific expression of EYFP in astrocytes (d, f; grey). g-h, Close-up view of 3D-max projection AT images showing Bassoon (red) before (g) and after (h) image processing, revealing engulfed Bassoon-positive synaptic material by astrocytes (grey). i-j, Close-up view of 3D-max projection AT images showing PSD-95 (cyan) before (i) and after (j) image processing, revealing engulfed PSD-95-positive synaptic material by astrocytes (grey). Scale bar, 50 μm (a) and 20 μm (b-j).
Similar articles
- Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis.
Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY, Park H, Chung WS. Lee JH, et al. Nature. 2021 Feb;590(7847):612-617. doi: 10.1038/s41586-020-03060-3. Epub 2020 Dec 23. Nature. 2021. PMID: 33361813 - Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex.
Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, Cirelli C. Bellesi M, et al. J Neurosci. 2017 May 24;37(21):5263-5273. doi: 10.1523/JNEUROSCI.3981-16.2017. J Neurosci. 2017. PMID: 28539349 Free PMC article. - Emerging evidence of context-dependent synapse elimination by phagocytes in the CNS.
Shen FS, Liu C, Sun HZ, Chen XY, Xue Y, Chen L. Shen FS, et al. J Leukoc Biol. 2024 Sep 2;116(3):511-522. doi: 10.1093/jleuko/qiae098. J Leukoc Biol. 2024. PMID: 38700080 Review. - Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis.
Byun YG, Kim NS, Kim G, Jeon YS, Choi JB, Park CW, Kim K, Jang H, Kim J, Kim E, Han YM, Yoon KJ, Lee SH, Chung WS. Byun YG, et al. Immunity. 2023 Sep 12;56(9):2105-2120.e13. doi: 10.1016/j.immuni.2023.07.005. Epub 2023 Jul 31. Immunity. 2023. PMID: 37527657 - Astrocyte Regulation of Synapse Formation, Maturation, and Elimination.
Chung WS, Baldwin KT, Allen NJ. Chung WS, et al. Cold Spring Harb Perspect Biol. 2024 Aug 1;16(8):a041352. doi: 10.1101/cshperspect.a041352. Cold Spring Harb Perspect Biol. 2024. PMID: 38346858 Review.
Cited by
- Glial cells as a promising therapeutic target of glaucoma: beyond the IOP.
Shinozaki Y, Namekata K, Guo X, Harada T. Shinozaki Y, et al. Front Ophthalmol (Lausanne). 2024 Jan 8;3:1310226. doi: 10.3389/fopht.2023.1310226. eCollection 2023. Front Ophthalmol (Lausanne). 2024. PMID: 38983026 Free PMC article. Review. - Astrocytic Factors Controlling Synaptogenesis: A Team Play.
Fossati G, Matteoli M, Menna E. Fossati G, et al. Cells. 2020 Sep 26;9(10):2173. doi: 10.3390/cells9102173. Cells. 2020. PMID: 32993090 Free PMC article. Review. - Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo.
Damisah EC, Hill RA, Rai A, Chen F, Rothlin CV, Ghosh S, Grutzendler J. Damisah EC, et al. Sci Adv. 2020 Jun 26;6(26):eaba3239. doi: 10.1126/sciadv.aba3239. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32637606 Free PMC article. - Essential Role of Astrocytes in Learning and Memory.
Escalada P, Ezkurdia A, Ramírez MJ, Solas M. Escalada P, et al. Int J Mol Sci. 2024 Feb 5;25(3):1899. doi: 10.3390/ijms25031899. Int J Mol Sci. 2024. PMID: 38339177 Free PMC article. Review.
References
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi:10.1016/j.neuron.2008.10.013. - PubMed
- MacDonald JM, et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi:10.1016/j.neuron.2006.04.028. - PubMed
- Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS069375/NS/NINDS NIH HHS/United States
- R01 NS075252/NS/NINDS NIH HHS/United States
- P30 NS069375/NS/NINDS NIH HHS/United States
- T32 MH020016/MH/NIMH NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- 5 R21NS072556/NS/NINDS NIH HHS/United States
- R21 NS072556/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous