GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy - PubMed (original) (raw)
Case Reports
doi: 10.1002/ana.24073. Epub 2014 Jan 2.
Rik Hendrickx, Kirsten Geider, Bodo Laube, Michael Schwake, Robert J Harvey, Victoria M James, Alex Pepler, Isabelle Steiner, Konstanze Hörtnagel, John Neidhardt, Susanne Ruf, Markus Wolff, Deborah Bartholdi, Roberto Caraballo, Konrad Platzer, Arvid Suls, Peter De Jonghe, Saskia Biskup, Sarah Weckhuysen
Affiliations
- PMID: 24272827
- PMCID: PMC4223934
- DOI: 10.1002/ana.24073
Case Reports
GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy
Johannes R Lemke et al. Ann Neurol. 2014 Jan.
Abstract
Objective: To identify novel epilepsy genes using a panel approach and describe the functional consequences of mutations.
Methods: Using a panel approach, we screened 357 patients comprising a vast spectrum of epileptic disorders for defects in genes known to contribute to epilepsy and/or intellectual disability (ID). After detection of mutations in a novel epilepsy gene, we investigated functional effects in Xenopus laevis oocytes and screened a follow-up cohort.
Results: We revealed de novo mutations in GRIN2B encoding the NR2B subunit of the N-methyl-D-aspartate (NMDA) receptor in 2 individuals with West syndrome and severe developmental delay as well as 1 individual with ID and focal epilepsy. The patient with ID and focal epilepsy had a missense mutation in the extracellular glutamate-binding domain (p.Arg540His), whereas both West syndrome patients carried missense mutations within the NR2B ion channel-forming re-entrant loop (p.Asn615Ile, p.Val618Gly). Subsequent screening of 47 patients with unexplained infantile spasms did not reveal additional de novo mutations, but detected a carrier of a novel inherited GRIN2B splice site variant in close proximity (c.2011-5_2011-4delTC). Mutations p.Asn615Ile and p.Val618Gly cause a significantly reduced Mg(2+) block and higher Ca(2+) permeability, leading to a dramatically increased Ca(2+) influx, whereas p.Arg540His caused less severe disturbance of channel function, corresponding to the milder patient phenotype.
Interpretation: We identified GRIN2B gain-of-function mutations as a cause of West syndrome with severe developmental delay as well as of ID with childhood onset focal epilepsy. Severely disturbed channel function corresponded to severe clinical phenotypes, underlining the important role of facilitated NMDA receptor signaling in epileptogenesis.
© 2014 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of Child Neurology Society/American Neurological Association.
Figures
Figure 1
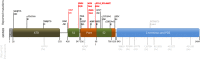
Location of GRIN2B mutations in a schematic illustration of the conserved domains of the NR2B subunit (SP = signal peptide; ATD = amino-terminal domain, involved in receptor assembly; S1 and S2 form the ligand-binding domain; Pore = re-entrant pore-forming and transmembrane spanning domains; PDZ = PDZ domain binding motif). All reported de novo mutations and their according phenotypes (ASD = autism spectrum disorders; FE = focal epilepsy; ID = intellectual disability; LGS = Lennox–Gastaut syndrome; Scz = schizophrenia) are listed in the top row. Mutations causing phenotypes without seizures are labeled in black, mutations in epilepsy patients are in red. So far, no pathogenic variants have been observed in the C-terminal region of NR2B. Mutations causing West syndrome cluster within re-entrant pore-forming domain, whereas the mutation causing ID and focal epilepsy was observed in the glutamate-binding domain S1, similar to a recently described LGS case. Nonsynonymous variants that are believed not to be associated with abnormal phenotypes (gray) and are reported more than once (in brackets) in the Exome Variant Server (EVS) are listed in the bottom line.
Figure 2
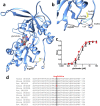
Structural and functional consequences of missense mutations in GRIN2B. (A) Topology model of an NR1 and an NR2B subunit. Positions of the alterations p.Arg540His, p.Asn615Ile and p.Val618Gly are indicated by asterisks in the NR2 subunit consisting of an amino-terminal domain (ATD), the ligand-binding domain (LBD) including the S1 and S2 peptide segments, 3 transmembrane segments (M1, M2, and M3), a re-entrant pore loop (P), and an intracellular carboxy-terminal domain (CTD). Residue Arg540 lies within the glutamate-binding domain, and Asn615 and Val618 in the ion channel pore. N = NH2-terminus; C = COOH-terminus. (B) Model of the transmembrane arrangement of the N-methyl-D-aspartate (NMDA) receptor composed of NR1 (green) and NR2B (cyan) subunits (top view). The arrow highlights the side chains of p.Asn615Ile and p.Val618Gly in the pore-forming region. (C) Gradual loss of Mg2+ inhibition of NR1-NR2B wild-type and NR1-NR2B mutant receptor currents at −70mV. Respective sample traces of NR1-NR2B and NR1-NR2BAsn615Ile are shown above with inhibition of receptor currents by 1mM Mg2+ of NR1-NR2B (96 ± 0.9%, n=4) and mutant NR1-NR2BAsn615Ile (14 ± 7.2%, p < 0.0001, n = 3), NR1-NR2BVal618Gly (48 ± 6.5%, _p_ = 0.0003, n = 3), and NR1-NR2BArg540His (81 ± 3.2%, _p_= 0.005, n = 5) receptors. (**D**) Effect on Ca2+permeability of NR1-NR2B wild-type and NR1-NR2B mutant receptor currents. Current–voltage relationships of NR1-NR2B receptors in the absence of Mg2+ in Na+-free extracellular solution reveal significant differences in the reversal potential (indicated by _arrows_) of NR1-NR2B (−31 ± 1.7mV, n = 4, _black triangles_) and mutant NR1-NR2BAsn615Ile (−1.0 ± 6.8mV,_p_ = 0.004, n = 3, _red squares_), NR1-NR2BVal618Gly (−5.4 ± 3.7mV, _p_ < 0.001, n = 3, _green squares_), and NR1-NR2BArg540His (−9.4 ± 6.5mV, _p_ = 0.013, n = 3, _blue squares_) receptor currents. (NMDG-Cl, N-methyl-D-glucamine chloride) Calculation of the relative divalent to monovalent cation permeability _P_Ca/_P_Na by the Goldman–Hodgkin–Katz voltage equation revealed a >3-fold increase in Ca2+ permeability of the mutant NMDA receptors (_P_Ca/_P_Na for NR1-NR2B = 0.86; NR1-NR2BAsn615Ile= 5.22; NR2BVal618Gly = 3.12; and NR2BArg540His = 3.23).
Figure 3
Structural and functional analyses of the glutamate binding-domain mutation Arg540His. (A) Residue 540 is predicted to be located within the glutamate-binding S1 domain, and is significant in the stabilization of the tertiary structure of the glutamate-binding domain. (B) Substitution p.Arg540His is likely to abolish hydrogen bonding (blue lines) with the backbone of Cys746 and His802 and a cation–pi interaction with His802, possibly leading to a relaxed fold in this region. (C) Pharmacological characterization of the apparent agonist affinities of wild-type NR1-NR2B (black triangles) and mutant NR1-NR2BArg540His (red squares) N-methyl-D-aspartate receptors measured after heterologous expression in Xenopus laevis oocytes by 2-electrode voltage-clamping revealed that similar glutamate concentrations were required to elicit half-maximal responses (EC50 values = 0.72 ± 0.22μM and 0.31 ± 0.02μM, respectively,p = 0.14, n = 3). (D) Arg540 is a highly conserved residue within NR2A–D subunits.
Similar articles
- Functional Evaluation of a Novel GRIN2B Missense Variant Associated with Epilepsy and Intellectual Disability.
Wang X, Mei D, Gou L, Zhao S, Gao C, Guo J, Luo S, Guo B, Yang Z, Wang Q, Tan T, Zhang Y. Wang X, et al. Neuroscience. 2023 Aug 21;526:107-120. doi: 10.1016/j.neuroscience.2023.06.018. Epub 2023 Jun 28. Neuroscience. 2023. PMID: 37385334 - Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes.
Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortüm F, Fritsch A, Pientka FK, Hellenbroich Y, Kalscheuer VM, Kohlhase J, Moog U, Rappold G, Rauch A, Ropers HH, von Spiczak S, Tönnies H, Villeneuve N, Villard L, Zabel B, Zenker M, Laube B, Reis A, Wieczorek D, Van Maldergem L, Kutsche K. Endele S, et al. Nat Genet. 2010 Nov;42(11):1021-6. doi: 10.1038/ng.677. Epub 2010 Oct 3. Nat Genet. 2010. PMID: 20890276 - A de novo loss-of-function GRIN2A mutation associated with childhood focal epilepsy and acquired epileptic aphasia.
Gao K, Tankovic A, Zhang Y, Kusumoto H, Zhang J, Chen W, XiangWei W, Shaulsky GH, Hu C, Traynelis SF, Yuan H, Jiang Y. Gao K, et al. PLoS One. 2017 Feb 9;12(2):e0170818. doi: 10.1371/journal.pone.0170818. eCollection 2017. PLoS One. 2017. PMID: 28182669 Free PMC article. - MED13 mutation: A novel cause of developmental and epileptic encephalopathy with infantile spasms.
Trivisano M, De Dominicis A, Micalizzi A, Ferretti A, Dentici ML, Terracciano A, Calabrese C, Vigevano F, Novelli G, Novelli A, Specchio N. Trivisano M, et al. Seizure. 2022 Oct;101:211-217. doi: 10.1016/j.seizure.2022.09.002. Epub 2022 Sep 3. Seizure. 2022. PMID: 36087421 Review. - De novo GRIN2A variants associated with epilepsy and autism and literature review.
Mangano GD, Riva A, Fontana A, Salpietro V, Mangano GR, Nobile G, Orsini A, Iacomino M, Battini R, Astrea G, Striano P, Nardello R. Mangano GD, et al. Epilepsy Behav. 2022 Apr;129:108604. doi: 10.1016/j.yebeh.2022.108604. Epub 2022 Feb 23. Epilepsy Behav. 2022. PMID: 35217385 Review.
Cited by
- Disease-Associated Variants in GRIN1, GRIN2A and GRIN2B genes: Insights into NMDA Receptor Structure, Function, and Pathophysiology.
Korinek M, Candelas Serra M, Abdel Rahman F, Dobrovolski M, Kuchtiak V, Abramova V, Fili K, Tomovic E, Hrcka Krausova B, Krusek J, Cerny J, Vyklicky L, Balik A, Smejkalova T. Korinek M, et al. Physiol Res. 2024 May 31;73(Suppl 1):S413-S434. doi: 10.33549/physiolres.935346. Epub 2024 May 31. Physiol Res. 2024. PMID: 38836461 Free PMC article. Review. - Rescuing tri-heteromeric NMDA receptor function: the potential of pregnenolone-sulfate in loss-of-function GRIN2B variants.
Kellner S, Berlin S. Kellner S, et al. Cell Mol Life Sci. 2024 May 25;81(1):235. doi: 10.1007/s00018-024-05243-x. Cell Mol Life Sci. 2024. PMID: 38795169 Free PMC article. - Anterior cingulate cortex-related functional hyperconnectivity underlies sensory hypersensitivity in Grin2b-mutant mice.
Lee S, Jung WB, Moon H, Im GH, Noh YW, Shin W, Kim YG, Yi JH, Hong SJ, Jung Y, Ahn S, Kim SG, Kim E. Lee S, et al. Mol Psychiatry. 2024 Oct;29(10):3195-3207. doi: 10.1038/s41380-024-02572-y. Epub 2024 May 4. Mol Psychiatry. 2024. PMID: 38704508 Free PMC article. - Genetic Advancements in Infantile Epileptic Spasms Syndrome and Opportunities for Precision Medicine.
Snyder HE, Jain P, RamachandranNair R, Jones KC, Whitney R. Snyder HE, et al. Genes (Basel). 2024 Feb 21;15(3):266. doi: 10.3390/genes15030266. Genes (Basel). 2024. PMID: 38540325 Free PMC article. Review. - ZFHX3 variants cause childhood partial epilepsy and infantile spasms with favourable outcomes.
He MF, Liu LH, Luo S, Wang J, Guo JJ, Wang PY, Zhai QX, He SL, Zou DF, Liu XR, Li BM, Ma HY, Qiao JD, Zhou P, He N, Yi YH, Liao WP. He MF, et al. J Med Genet. 2024 Jun 20;61(7):652-660. doi: 10.1136/jmg-2023-109725. J Med Genet. 2024. PMID: 38508705 Free PMC article.
References
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. - PubMed
- Roger J. Epileptic syndromes in infancy, childhood and adolescence. Montrouge, France: John Libbey Eurotext; 2005.
- Stromme P, Mangelsdorf ME, Scheffer IE, Gecz J. Infantile spasms, dystonia, and other X-linked phenotypes caused by mutations in Aristaless related homeobox gene, ARX. Brain Dev. 2002;24:266–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous