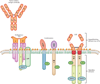Chimeric antigen receptor therapy for cancer - PubMed (original) (raw)
Review
Chimeric antigen receptor therapy for cancer
David M Barrett et al. Annu Rev Med. 2014.
Abstract
Improved outcomes for patients with cancer hinge on the development of new targeted therapies with acceptable short-term and long-term toxicity. Progress in basic, preclinical, and clinical arenas spanning cellular immunology, synthetic biology, and cell-processing technologies has paved the way for clinical applications of chimeric antigen receptor-based therapies. This new form of targeted immunotherapy merges the exquisite targeting specificity of monoclonal antibodies with the potent cytotoxicity and long-term persistence provided by cytotoxic T cells. Although this field is still in its infancy, clinical trials have already shown clinically significant antitumor activity in neuroblastoma, chronic lymphocytic leukemia, and B cell lymphoma, and trials targeting a variety of other adult and pediatric malignancies are under way. Ongoing work is focused on identifying optimal tumor targets and on elucidating and manipulating both cell- and host-associated factors to support expansion and persistence of the genetically engineered cells in vivo. The potential to target essentially any tumor-associated cell-surface antigen for which a monoclonal antibody can be made opens up an entirely new arena for targeted therapy of cancer.
Conflict of interest statement
DISCLOSURE STATEMENT
The University of Pennsylvania has entered into a partnership with Novartis for the development of chimeric antigen receptors. This partnership is managed in accordance with the University of Pennsylvania’s Conflict of Interest Policy. All authors are in compliance with this policy.
Figures
Figure 1
Antibodies can bind to surface antigens expressed on tumor cells. Chimeric antigen receptors (CARs) have a single-chain antibody fragment (scFv), expressed in tandem with signaling elements derived from the T cell receptor (TCR) and costimulatory domains such as 4-1BB and CD28.
Figure 2
Chimeric antigen receptor (CAR) therapy is similar to an autologous bone marrow transplantation procedure. T cells are collected from the patient by apheresis, and the T cells are expanded and genetically modified using several approaches before they are returned to the patient. Abbreviation: APCs, antigen-presenting cells.
Similar articles
- The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer.
Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA. Lee DW, et al. Clin Cancer Res. 2012 May 15;18(10):2780-90. doi: 10.1158/1078-0432.CCR-11-1920. Clin Cancer Res. 2012. PMID: 22589486 Free PMC article. - Chimeric antigen receptor engineering: a right step in the evolution of adoptive cellular immunotherapy.
Figueroa JA, Reidy A, Mirandola L, Trotter K, Suvorava N, Figueroa A, Konala V, Aulakh A, Littlefield L, Grizzi F, Rahman RL, Jenkins MR, Musgrove B, Radhi S, D'Cunha N, D'Cunha LN, Hermonat PL, Cobos E, Chiriva-Internati M. Figueroa JA, et al. Int Rev Immunol. 2015 Mar;34(2):154-87. doi: 10.3109/08830185.2015.1018419. Int Rev Immunol. 2015. PMID: 25901860 Review. - Prospects for personalized combination immunotherapy for solid tumors based on adoptive cell therapies and immune checkpoint blockade therapies.
Kato D, Yaguchi T, Iwata T, Morii K, Nakagawa T, Nishimura R, Kawakami Y. Kato D, et al. Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(1):68-77. doi: 10.2177/jsci.40.68. Nihon Rinsho Meneki Gakkai Kaishi. 2017. PMID: 28539557 Review. - Chimeric Antigen Receptor T Cell Therapy in Hematology.
Ataca P, Arslan Ö. Ataca P, et al. Turk J Haematol. 2015 Dec;32(4):285-94. doi: 10.4274/tjh.2015.0049. Epub 2015 Aug 6. Turk J Haematol. 2015. PMID: 26377367 Free PMC article. Review. - [T Lymphocytes with Modified Specificity in the Therapy of Malignant Diseases].
Vdovin AS, Bykova NA, Efimov GA. Vdovin AS, et al. Mol Biol (Mosk). 2017 Nov-Dec;51(6):1008-1023. doi: 10.7868/S002689841706012X. Mol Biol (Mosk). 2017. PMID: 29271964 Review. Russian.
Cited by
- IL-6 Blockade in Cytokine Storm Syndromes.
Barrett D. Barrett D. Adv Exp Med Biol. 2024;1448:565-572. doi: 10.1007/978-3-031-59815-9_37. Adv Exp Med Biol. 2024. PMID: 39117839 Review. - Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8(+) T cells.
Rubinstein MP, Su EW, Suriano S, Cloud CA, Andrijauskaite K, Kesarwani P, Schwartz KM, Williams KM, Johnson CB, Li M, Scurti GM, Salem ML, Paulos CM, Garrett-Mayer E, Mehrotra S, Cole DJ. Rubinstein MP, et al. Cancer Immunol Immunother. 2015 May;64(5):539-49. doi: 10.1007/s00262-015-1655-y. Epub 2015 Feb 13. Cancer Immunol Immunother. 2015. PMID: 25676709 Free PMC article. - Stem-cell Based Engineered Immunity Against HIV Infection in the Humanized Mouse Model.
Zhen A, Rezek V, Youn C, Rick J, Lam B, Chang N, Zack J, Kamata M, Kitchen S. Zhen A, et al. J Vis Exp. 2016 Jul 2;(113):54048. doi: 10.3791/54048. J Vis Exp. 2016. PMID: 27404517 Free PMC article. - Chemical Tools To Monitor and Manipulate Adaptive Immune Responses.
Doran TM, Sarkar M, Kodadek T. Doran TM, et al. J Am Chem Soc. 2016 May 18;138(19):6076-94. doi: 10.1021/jacs.6b02954. Epub 2016 May 3. J Am Chem Soc. 2016. PMID: 27115249 Free PMC article. Review. - Novel chimeric antigen receptor T cells based on T-cell receptor-like antibodies.
Zhao Q. Zhao Q. Blood Sci. 2019 Oct 21;1(2):144-147. doi: 10.1097/BS9.0000000000000032. eCollection 2019 Oct. Blood Sci. 2019. PMID: 35402807 Free PMC article.
References
- Barnes DW, Ford CE, Ilbery PL, et al. Tissue transplantation in the radiation chimera. J. Cell. Physiol. Suppl. 1957;50:123–138. - PubMed
- Barnes DW, Loutit JF. Treatment of murine leukaemia with x-rays and homologous bone marrow. II. Br. J. Haematol. 1957;3:241–252. - PubMed
- Mathe G, Amiel JL, Schwarzenberg L, et al. Adoptive immunotherapy of acute leukemia: experimental and clinical results. Cancer Res. 1965;25:1525–1531. - PubMed
- Rosenberg SA, Terry WD. Passive immunotherapy of cancer in animals and man. Adv. Cancer Res. 1977;25:323–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA066726/CA/NCI NIH HHS/United States
- R01 CA116660/CA/NCI NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- T32 CA009615/CA/NCI NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- R01 CA102646/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources