Active demethylation in mouse zygotes involves cytosine deamination and base excision repair - PubMed (original) (raw)
Active demethylation in mouse zygotes involves cytosine deamination and base excision repair
Fátima Santos et al. Epigenetics Chromatin. 2013.
Abstract
Background: DNA methylation in mammals is an epigenetic mark necessary for normal embryogenesis. During development active loss of methylation occurs in the male pronucleus during the first cell cycle after fertilisation. This is accompanied by major chromatin remodelling and generates a marked asymmetry between the paternal and maternal genomes. The mechanism(s) by which this is achieved implicate, among others, base excision repair (BER) components and more recently a major role for TET3 hydroxylase. To investigate these methylation dynamics further we have analysed DNA methylation and hydroxymethylation in fertilised mouse oocytes by indirect immunofluorescence (IF) and evaluated the relative contribution of different candidate factors for active demethylation in knock-out zygotes by three-dimensional imaging and IF semi-quantification.
Results: We find two distinct phases of loss of paternal methylation in the zygote, one prior to and another coincident with, but not dependent on, DNA replication. TET3-mediated hydroxymethylation is limited to the replication associated second phase of demethylation. Analysis of cytosine deaminase (AID) null fertilised oocytes revealed a role for this enzyme in the second phase of loss of paternal methylation, which is independent from hydroxymethylation. Investigation into the possible repair pathways involved supports a role for AID-mediated cytosine deamination with subsequent U-G mismatch long-patch BER by UNG2 while no evidence could be found for an involvement of TDG.
Conclusions: There are two observable phases of DNA demethylation in the mouse zygote, before and coincident with DNA replication. TET3 is only involved in the second phase of loss of methylation. Cytosine deamination and long-patch BER mediated by UNG2 appear to independently contribute to this second phase of active demethylation. Further work will be necessary to elucidate the mechanism(s) involved in the first phase of active demethylation that will potentially involve activities required for early sperm chromatin remodelling.
Figures
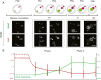
Figure 1
Paternal loss of DNA methylation occurs in two phases. (A) Diagrammatic illustration and representative two-dimensional (2D) projections of Z-stack images of control (B6 x B6) pronuclear stage embryos (PN0 to PN5) simultaneously stained for DNA methylation (5mC) and hydroxymethylation (5hmC) clearly showing two phases of paternal loss of methylation, Phase I, corresponding to a pre-replicative state with no observable change in DNA hydroxymethylation, and Phase II, when DNA replication is taking place, and during which a very significant increase in DNA hydroxymethylation in the paternal pronucleus takes place. Scale bar 25 μm. f, female pronucleus; m, male pronucleus; pb, polar body. (B) The dynamic changes of DNA methylation and hydroxymethylation during the first cell cycle can be represented by the ratio of the total immunofluorescence signal (3D imaging) between the maternal and paternal pronuclei (male/female ratio) for 5mC and 5hmC, respectively. Values at time of fertilisation are hypothetical (dashed lines), calculated considering a minimum initial total 5mC and 5hmC for sperm and oocytes (see text for explanation). Values plotted for stages between PN1 and PN5 (minimum 10 embryos per stage). Bars indicate standard deviation.
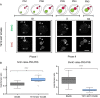
Figure 2
TET3 null fertilised oocytes show increased paternal DNA methylation and reduced hydroxymethylation. (A) Diagrammatic illustration and representative 2D projections of Z-stack images of TET3 maternally deleted oocytes fertilised by control sperm (TET3 MAT KOxB6) pronuclear stage embryos (PN1 to PN5) simultaneously stained for DNA methylation (5mC) and hydroxymethylation (5hmC) clearly showing paternal loss of methylation during Phase I, corresponding to a pre-replicative state, but no apparent further demethylation during Phase II, when DNA replication is taking place, and during which there is complete failure in TET3 null oocytes to generate DNA hydroxymethylation in the paternal pronucleus. Scale bar 25 μm. f, female pronucleus; m, male pronucleus; pb, polar body. (B) Comparison of the changes of DNA methylation and hydroxymethylation between control (B6xB6; Figure 1A) and TET3 maternally deleted (TET3 MAT KOxB6) mid-late zygotes. Box-and-whisker plots of the total immunofluorescence signal (3D imaging semi-quantification) ratio between the paternal and maternal pronuclei (male/female ratio) for 5mC and 5hmC, respectively, showing, on the left, a very significant increase in the levels of paternal DNA methylation and, on the right, an equally significant decrease in the levels of hydroxymethylation in TET3 maternally deleted zygotes compared to controls. ****(P <0.0001, two-tailed Mann–Whitney test).
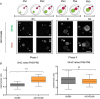
Figure 3
AID null fertilised oocytes show increased paternal DNA methylation but equal levels of hydroxymethylation. (A) Diagrammatic illustration and representative 2D projections of Z-stack images of AID null oocytes fertilised by control sperm (AID KOxB6) pronuclear stage embryos (PN1 to PN5) simultaneously stained for DNA methylation (5mC) and hydroxymethylation (5hmC) clearly showing paternal loss of methylation during Phase I, corresponding to a pre-replicative state, but no apparent further demethylation during Phase II, when DNA replication is taking place, while showing a striking increase in DNA hydroxymethylation in the paternal pronucleus, similar to that observed in control (B6xB6) embryos. Scale bar 25 μm. f, female pronucleus; m, male pronucleus; pb, polar body. (B) Comparison of the changes of DNA methylation and hydroxymethylation between control (B6xB6) and AID null (AID KOxB6) mid-late zygotes. Box-and-whisker plots of the total immunofluorescence signal (3D imaging semi-quantification) ratio between the paternal and maternal pronuclei (male/female ratio) for 5mC and 5hmC, respectively, showing, on the left, a very significant increase in the levels of paternal DNA methylation but no significant difference in the levels of hydroxymethylation in AID null zygotes compared to controls, on the right. ****(P <0.0001, two-tailed Mann–Whitney test), ns (P = 0.6330, two-tailed unpaired t test with Welch’s correction).
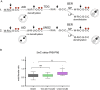
Figure 4
Evidence supports AID mediated cytosine deamination with U-G mismatch long-patch BER in the zygote. (A) Schematic representation of the possible AID-mediated deamination scenarios. (1) AID deamination of a methylated cytosine in the context of the preferred binding motif (WRC) resulting in demethylation and creating a T-G mismatch. This would be recognised by TDG generating and apyrimidinic site that would subsequently be repaired by either short-patch (SP) (no further loss of methylation in neighbouring methylated cytosines) or long-patch (LP) (possible further demethylation by new incorporation of cytosines not followed by de novo methylation) BER. (2) AID deamination of a non-methylated cytosine in the context of the preferred binding motif (WRC) resulting in no loss of methylation and creating a U-G mismatch. This would be recognised by UNG2 generating and apurinic site that would subsequently be repaired by either SP (no loss of methylation in neighbouring methylated cytosines) or LP (resulting in demethylation by new incorporation of cytosines not followed by de novo methylation) BER. (B) Comparison of the changes of DNA methylation between wild-type control (B6xB6), TDG maternally deleted (TDG MAT KOxB6) and UNG2 null (UNG2 KOxB6) late zygotes. Box-and-whisker plots of the total immunofluorescence signal (3D imaging semi-quantification) ratio between the paternal and maternal pronuclei (male/female ratio) for 5mC showing there is only a significant increase in the levels of paternal DNA methylation in UNG2 deleted and not in TDG maternally deleted fertilised oocytes. This is compatible with AID-mediated cytosine deamination followed by LP BER with no evidence for direct 5mC deamination and T-G mismatch repair, which is in agreement with the reported reduced activity of AID on 5mC relative to cytosine, its canonical substrate. **(P < 0.05, ANOVA, Dunn’s multiple comparison test), ns (_P_ > 0.05, ANOVA, Dunn’s multiple comparison test).
Similar articles
- Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes.
Shen L, Inoue A, He J, Liu Y, Lu F, Zhang Y. Shen L, et al. Cell Stem Cell. 2014 Oct 2;15(4):459-471. doi: 10.1016/j.stem.2014.09.002. Cell Stem Cell. 2014. PMID: 25280220 Free PMC article. - Dynamics of 5-methylcytosine and 5-hydroxymethylcytosine during pronuclear development in equine zygotes produced by ICSI.
Heras S, Smits K, De Schauwer C, Van Soom A. Heras S, et al. Epigenetics Chromatin. 2017 Mar 15;10:13. doi: 10.1186/s13072-017-0120-x. eCollection 2017. Epigenetics Chromatin. 2017. PMID: 28331549 Free PMC article. - The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes.
Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabó PE, Pfeifer GP, Li J, Xu GL. Gu TP, et al. Nature. 2011 Sep 4;477(7366):606-10. doi: 10.1038/nature10443. Nature. 2011. PMID: 21892189 - DNA demethylation pathways: Additional players and regulators.
Bochtler M, Kolano A, Xu GL. Bochtler M, et al. Bioessays. 2017 Jan;39(1):1-13. doi: 10.1002/bies.201600178. Epub 2016 Nov 16. Bioessays. 2017. PMID: 27859411 Review. - Cytosine methylation and DNA repair.
Walsh CP, Xu GL. Walsh CP, et al. Curr Top Microbiol Immunol. 2006;301:283-315. doi: 10.1007/3-540-31390-7_11. Curr Top Microbiol Immunol. 2006. PMID: 16570853 Review.
Cited by
- DNA dioxygenases Tet2/3 regulate gene promoter accessibility and chromatin topology in lineage-specific loci to control epithelial differentiation.
Chen GD, Fatima I, Xu Q, Rozhkova E, Fessing MY, Mardaryev AN, Sharov AA, Xu GL, Botchkarev VA. Chen GD, et al. Sci Adv. 2023 Jan 13;9(2):eabo7605. doi: 10.1126/sciadv.abo7605. Epub 2023 Jan 11. Sci Adv. 2023. PMID: 36630508 Free PMC article. - Localization Matters: Epigenetic Regulation of Natural Killer Cells in Different Tissue Microenvironments.
Wiedemann GM. Wiedemann GM. Front Immunol. 2022 May 30;13:913054. doi: 10.3389/fimmu.2022.913054. eCollection 2022. Front Immunol. 2022. PMID: 35707540 Free PMC article. Review. - Reprogramming the methylome: erasing memory and creating diversity.
Lee HJ, Hore TA, Reik W. Lee HJ, et al. Cell Stem Cell. 2014 Jun 5;14(6):710-9. doi: 10.1016/j.stem.2014.05.008. Cell Stem Cell. 2014. PMID: 24905162 Free PMC article. - A Surveillance Mechanism Ensures Repair of DNA Lesions during Zygotic Reprogramming.
Ladstätter S, Tachibana-Konwalski K. Ladstätter S, et al. Cell. 2016 Dec 15;167(7):1774-1787.e13. doi: 10.1016/j.cell.2016.11.009. Epub 2016 Dec 1. Cell. 2016. PMID: 27916276 Free PMC article. - Gender Differences in Global but Not Targeted Demethylation in iPSC Reprogramming.
Milagre I, Stubbs TM, King MR, Spindel J, Santos F, Krueger F, Bachman M, Segonds-Pichon A, Balasubramanian S, Andrews SR, Dean W, Reik W. Milagre I, et al. Cell Rep. 2017 Jan 31;18(5):1079-1089. doi: 10.1016/j.celrep.2017.01.008. Cell Rep. 2017. PMID: 28147265 Free PMC article.
References
- Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;6:501–502. - PubMed
- Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun. 2011;6:241. - PubMed
Grants and funding
- 095645/WT_/Wellcome Trust/United Kingdom
- BBS/E/B/0000H112/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0700098/MRC_/Medical Research Council/United Kingdom
- MC_U105178806/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous