Therapeutic depletion of natural killer cells controls persistent infection - PubMed (original) (raw)
Therapeutic depletion of natural killer cells controls persistent infection
Stephen N Waggoner et al. J Virol. 2014 Feb.
Abstract
Persistent viral infections are associated with host and viral factors that impair effective antiviral immunity. Natural killer (NK) cells contribute to establishment of persistent lymphocytic choriomeningitis virus (LCMV) infection in mice through suppression of virus-specific T cell responses during the first few days of infection, but NK cell depletion during those early time points can enable severe T cell-mediated immune pathology and death of the host. Here we show that long after their peak in cytolytic activation, NK cells continue to support viral persistence at later times of infection. Delayed depletion of NK cells, 2 to 3 weeks after infection, enhanced virus-specific T cell responses and viral control. This enhancing effect of delayed NK cell depletion on antiviral immunity, in contrast to early NK cell depletion, was not associated with increased morbidity and mortality, and mice quickly regained weight after treatment. The efficacy of the depletion depended in part upon the size of the original virus inoculum, the viral load at the time of depletion, and the presence of CD4 T cells. Each of these factors is an important contributor to the degree of CD8 T cell dysfunction during viral persistence. Thus, NK cells may continuously contribute to exhaustion of virus-specific T cells during chronic infection, possibly by depleting CD4 T cells. Targeting of NK cells could thus be considered in combination with blockade of other immunosuppressive pathways, such as the interleukin-10 (IL-10) and programmed death 1 (PD-1) pathways, as a therapy to cure chronic human infections, including those with HIV or hepatitis C virus. IMPORTANCE Persistent virus infections are a major threat to global human health. The capacity of viruses, including HIV and hepatitis C virus, to overwhelm or subvert host immune responses contributes to a prolonged state of dampened antiviral immune functionality, which in turn facilitates viral persistence. Recent efforts have focused on therapeutics that can restore the effector functions of these functionally exhausted virus-specific T cells in order to expedite viral clearance. Here we establish that natural killer (NK) cells actively contribute to immune dysfunction and viral persistence at later stages of infection. This previously undescribed mechanism of immune suppression during chronic infection provides a vital clue for the design of novel therapeutic strategies targeting NK cell immunosuppressive activity in order to restore immune function and enhance viral control in chronically infected individuals.
Figures
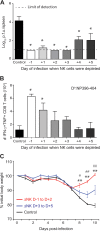
FIG 1
Timing of NK cell immunoregulatory activities during pathogenic medium-dose infection. C57BL/6 mice (n = 3/group) were treated with either NK cell-depleting anti-NK1.1 antibody or a nondepleting isotype control at various time points relative to i.v. inoculation with a medium dose (2 × 105 PFU) of LCMV clone 13. (A) At day 15 p.i., viral titers in the spleen were determined. Values below the lower limit of detection (log10 PFU = 1.0; dotted line) were assigned a “less than” value. *, P ≤ 0.05 versus nondepleted group. (B) Numbers of splenic IFN-γ+ TNF+ LCMV NP396–404-specific CD8 T cells were determined at day 15 p.i. by intracellular cytokine staining. *, P ≤ 0.05 versus nondepleted group. TNF, tumor necrosis factor. (C) Body weight was measured daily, and three patterns of weight loss were observed. The mean % of initial body weight was graphed as a function of time for nondepleted mice (black line; n = 3) and for pooled (phenotypically) groups of mice depleted at very early (day −1 [D−1], D+1, or D+2) (red line; n = 8) and later (D+3, D+4, or D+5) (blue line; n = 9) times of infection. **, P ≤ 0.01 versus nondepleted group; #, P ≤ 0.05 versus D+3 to D+5 group; ##, P ≤ 0.01 versus D+3 to D+5 group. Data are presented as means ± SEM and are representative of two independent experiments.
FIG 2
Therapeutic depletion of NK cells enhances control of persistent LCMV infection. Groups of C57BL/6 mice were inoculated i.v. with a high dose (2 × 106 PFU) of LCMV clone 13. (A and B) At days 10 and 13 p.i., mice were either treated with an isotype control antibody (n = 8) or depleted of NK cells (n = 9). (A) Viral titers in serum. (B) Body weights (n = 6/group) relative to weights prior to NK cell depletion on day 10 p.i. (C and D) Other groups of mice were treated with isotype control antibody or given a single injection of anti-NK1.1 antibody on days 20 and 21 (C) or days 28 to 31 (D) of infection. Viral titers in various tissues of isotype control (n = 9 or 10)- or anti-NK1.1 (n = 10)-treated mice were determined 10 to 13 (C) or 12 to 14 (D) days after NK cell depletion. Values below the limit of detection for the spleen (log10 PFU = 1) and other tissues (log10 PFU = 2) were assigned a “less than” value. Data in panels A, C, and D were pooled from two independent experiments. Data in panel B are representative of two similar experiments.
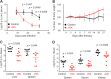
FIG 3
Therapeutic depletion of NK cells augments virus-specific T cell responses. Groups of C57BL/6 mice were inoculated i.v. with a high dose (2 × 106 PFU) of LCMV clone 13. At days 28 to 31 of infection, mice were either treated with isotype control antibody (n = 8) or depleted of NK cells (n = 9). Proportions (A) and numbers (B) of LCMV GP33–41-specific CD8 T cells in the blood and spleen were determined by intracellular cytokine staining after ex vivo stimulation with peptide or anti-CD3 antibody. (C) Numbers of IFN-γ+ CD4 T cells (n = 5/group) in the spleen. Data were pooled from two independent experiments.
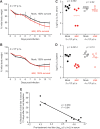
FIG 4
Effect of NK cell depletion is sensitive to size of LCMV inoculum. C57BL/6 mice (n = 3 or 4/group) were depleted of NK cells or treated with isotype control antibody 1 day prior to infection with a high (2 × 106 PFU) (A) or very high (4 × 106 PFU) (B) dose of LCMV clone 13 i.v. Body weight and survival were monitored for 11 days. (C and D) Mice (n = 5/group) were infected for 10 days with a high (2 × 106 PFU) or very high (4 × 106 PFU) dose of LCMV clone 13 prior to isotype control treatment or administration of anti-NK1.1 antibody. At day 31 p.i., 21 days after treatment, viral titers in the sera (C) and spleens (D) of these mice were determined. (E) For a group of mice infected with the high (2 × 106 PFU) dose of LCMV clone 13, viral titers in sera just prior to depletion of NK cells (day 10) were plotted against the % reduction in viral titers in sera 21 days later (endpoint viral titer/initial viral titer × 100 = % reduction), revealing an inverse relationship between these parameters.
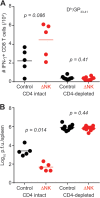
FIG 5
CD4 T cells are required for efficacy of NK cell depletion therapy. C57BL/6 mice were either depleted of CD4 T cells (n = 10/group) or treated with isotype control antibody (n = 5/group) at days −1 and +1 of high-dose LCMV clone 13 infection. At days 26 to 29 of infection, groups of mice were either treated with isotype control antibody or depleted of NK cells. LCMV-specific CD8 T cell responses (A) and viral titers (B) were measured in the spleen. These results were pooled from two independent experiments.
Similar articles
- Natural killer cells act as rheostats modulating antiviral T cells.
Waggoner SN, Cornberg M, Selin LK, Welsh RM. Waggoner SN, et al. Nature. 2011 Nov 20;481(7381):394-8. doi: 10.1038/nature10624. Nature. 2011. PMID: 22101430 Free PMC article. - Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity.
Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brüstle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Häussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS. Lang PA, et al. Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):1210-5. doi: 10.1073/pnas.1118834109. Epub 2011 Dec 13. Proc Natl Acad Sci U S A. 2012. PMID: 22167808 Free PMC article. - Adenovirus Vector Vaccination Impacts NK Cell Rheostat Function following Lymphocytic Choriomeningitis Virus Infection.
Blass E, Aid M, Martinot AJ, Larocca RA, Kang ZH, Badamchi-Zadeh A, Penaloza-MacMaster P, Reeves RK, Barouch DH. Blass E, et al. J Virol. 2018 May 14;92(11):e02103-17. doi: 10.1128/JVI.02103-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29514912 Free PMC article. - Immune Exhaustion: Past Lessons and New Insights from Lymphocytic Choriomeningitis Virus.
Kahan SM, Zajac AJ. Kahan SM, et al. Viruses. 2019 Feb 13;11(2):156. doi: 10.3390/v11020156. Viruses. 2019. PMID: 30781904 Free PMC article. Review. - The Good and the Bad of Natural Killer Cells in Virus Control: Perspective for Anti-HBV Therapy.
Fisicaro P, Rossi M, Vecchi A, Acerbi G, Barili V, Laccabue D, Montali I, Zecca A, Penna A, Missale G, Ferrari C, Boni C. Fisicaro P, et al. Int J Mol Sci. 2019 Oct 13;20(20):5080. doi: 10.3390/ijms20205080. Int J Mol Sci. 2019. PMID: 31614928 Free PMC article. Review.
Cited by
- NKp44/HLA-DP-dependent regulation of CD8 effector T cells by NK cells.
Padoan B, Casar C, Krause J, Schultheiss C, Baumdick ME, Niehrs A, Zecher BF, Pujantell M, Yuki Y, Martin M, Remmerswaal EBM, Dekker T, van der Bom-Baylon ND, Noble JA, Carrington M, Bemelman FJ, van Lier RAW, Binder M, Gagliani N, Bunders MJ, Altfeld M. Padoan B, et al. Cell Rep. 2024 Apr 23;43(4):114089. doi: 10.1016/j.celrep.2024.114089. Epub 2024 Apr 13. Cell Rep. 2024. PMID: 38615318 Free PMC article. - Hepatitis B Virus Reactivation in Cancer Patients Undergoing Immune Checkpoint Inhibitors Therapy: A Systematic Review.
Zhao J, Zhang Y, Qin S, Zou B, Wang Y. Zhao J, et al. J Cancer. 2022 Oct 31;13(14):3539-3553. doi: 10.7150/jca.77247. eCollection 2022. J Cancer. 2022. PMID: 36484006 Free PMC article. Review. - Burgeoning Exploration of the Role of Natural Killer Cells in Anti-PD-1/PD-L1 Therapy.
Bai R, Cui J. Bai R, et al. Front Immunol. 2022 May 12;13:886931. doi: 10.3389/fimmu.2022.886931. eCollection 2022. Front Immunol. 2022. PMID: 35634343 Free PMC article. Review. - Sustained Antibody-Dependent NK Cell Functions in Mild COVID-19 Outpatients During Convalescence.
Fuentes-Villalobos F, Garrido JL, Medina MA, Zambrano N, Ross N, Bravo F, Gaete-Argel A, Oyarzún-Arrau A, Amanat F, Soto-Rifo R, Valiente-Echeverría F, Ocampo R, Esveile C, Ferreira L, Cabrera J, Torres V, Rioseco ML, Riquelme R, Barría S, Alvarez R, Pinos Y, Krammer F, Calvo M, Barria MI; COVID-19 South Chile Group. Fuentes-Villalobos F, et al. Front Immunol. 2022 Feb 7;13:796481. doi: 10.3389/fimmu.2022.796481. eCollection 2022. Front Immunol. 2022. PMID: 35197972 Free PMC article. - Exhausted NK cells and cytokine storms in COVID-19: Whether NK cell therapy could be a therapeutic choice.
Ghasemzadeh M, Ghasemzadeh A, Hosseini E. Ghasemzadeh M, et al. Hum Immunol. 2022 Jan;83(1):86-98. doi: 10.1016/j.humimm.2021.09.004. Epub 2021 Sep 8. Hum Immunol. 2022. PMID: 34583856 Free PMC article. Review.
References
- Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, Oxenius A. 2012. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209:2485–2499. 10.1084/jem.20121015 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI081675/AI/NIAID NIH HHS/United States
- AI-17672/AI/NIAID NIH HHS/United States
- AI07349/AI/NIAID NIH HHS/United States
- R37 AI017672/AI/NIAID NIH HHS/United States
- U19 AI109858/AI/NIAID NIH HHS/United States
- T32 AI007349/AI/NIAID NIH HHS/United States
- CA34461/CA/NCI NIH HHS/United States
- R01 AI017672/AI/NIAID NIH HHS/United States
- R01 CA034461/CA/NCI NIH HHS/United States
- AI-081675/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials