A small molecule TrkB ligand reduces motor impairment and neuropathology in R6/2 and BACHD mouse models of Huntington's disease - PubMed (original) (raw)
A small molecule TrkB ligand reduces motor impairment and neuropathology in R6/2 and BACHD mouse models of Huntington's disease
Danielle A Simmons et al. J Neurosci. 2013.
Erratum in
- J Neurosci. 2014 Jan 29;34(5):2012
Abstract
Loss of neurotrophic support in the striatum caused by reduced brain-derived neurotrophic factor (BDNF) levels plays a critical role in Huntington's disease (HD) pathogenesis. BDNF acts via TrkB and p75 neurotrophin receptors (NTR), and restoring its signaling is a prime target for HD therapeutics. Here we sought to determine whether a small molecule ligand, LM22A-4, specific for TrkB and without effects on p75(NTR), could alleviate HD-related pathology in R6/2 and BACHD mouse models of HD. LM22A-4 was administered to R6/2 mice once daily (5-6 d/week) from 4 to 11 weeks of age via intraperitoneal and intranasal routes simultaneously to maximize brain levels. The ligand reached levels in the R6/2 forebrain greater than the maximal neuroprotective dose in vitro and corrected deficits in activation of striatal TrkB and its key signaling intermediates AKT, PLCγ, and CREB. Ligand-induced TrkB activation was associated with a reduction in HD pathologies in the striatum including decreased DARPP-32 levels, neurite degeneration of parvalbumin-containing interneurons, inflammation, and intranuclear huntingtin aggregates. Aggregates were also reduced in the cortex. Notably, LM22A-4 prevented deficits in dendritic spine density of medium spiny neurons. Moreover, R6/2 mice given LM22A-4 demonstrated improved downward climbing and grip strength compared with those given vehicle, though these groups had comparable rotarod performances and survival times. In BACHD mice, long-term LM22A-4 treatment (6 months) produced similar ameliorative effects. These results support the hypothesis that targeted activation of TrkB inhibits HD-related degenerative mechanisms, including spine loss, and may provide a disease mechanism-directed therapy for HD and other neurodegenerative conditions.
Figures
Figure 1.
LM22A-4 activates TrkB and its associated downstream signaling in striatum of R6/2 mice. Representative Western blots of striatal homogenates from 11- to 12-week-old WT or R6/2 mice treated with vehicle (Veh) or LM22A-4 (LM-4) for 7 weeks. For all quantitation, n = 8–10 mice/group were used and values were normalized to the WT Veh group run on the same gel. Immunobands from one mouse per group and corresponding densitometric group analyses are shown. A–D, Full-length TrkB levels (A) and its phosphorylation (p) at tyrosine residues Y705 (B), Y515 (C), and Y817 (D) are depicted. Samples from each mouse were run in three to four replicates. TrkB levels were lower in striatum of R6/2 mice relative to WTs (***p ≤ 0.001 vs WT Veh) regardless of LM-4 treatment. TrkB phosphorylation (pTrkB) at Y705 (**p ≤ 0.01 vs WT Veh), but not Y515 or Y817, was decreased in Veh-treated R6/2 mice compared with WTs. LM-4 inhibited the reduction in Y705 phosphorylation (+p ≤ 0.05 vs R6/2 Veh). LM-4 also increased phosphorylation at Y515 in WT mice (#p ≤ 0.05 vs WT Veh; two-tailed Student's t test). E–G, Western blots showing phosphorylation of AKT (E), ERK (F), and PLCγ (G), the three main downstream signaling pathways of TrkB. Samples from each mouse were run in four to six replicates. Phosphorylation of AKT, PLCγ, and ERK was decreased in Veh-treated R6/2 mice compared with WTs (**p ≤ 0.01 and ***p ≤ 0.001 vs WT Veh). LM-4 prevented the deficits in AKT and PLCγ (+p ≤ 0.05 and ++p ≤ 0.01 vs R6/2 Veh) but not ERK phosphorylation. H, Western blot showing phosphorylation of CREB at Ser133. Samples from each mouse were run in duplicate. pCREB was reduced by 36 ± 10% in R6/2 mice given Veh (*p < 0.05 vs WT Veh; two-tailed Student's t test) and LM-4 prevented this decrease (+p < 0.05 vs R6/2 Veh; two-tailed Student's t test); there was also an increase in pCREB in the WT LM-4 group that did not reach statistical significance (p = 0.07 vs WT Veh). I, Western blot showing mBDNF protein levels in striatum. Samples from each mouse were run in triplicate. mBDNF levels are reduced in striatum of R6/2 Veh mice (***p ≤ 0.001 vs WT Veh) and this difference was attenuated by LM-4 treatment (+p ≤ 0.05 vs R6/2 Veh; two-tailed Student's t test). For all analyses, results are expressed as the mean ± SEM and, for comparison purposes, some nonadjacent lanes of Western blots were moved together and separated by spaces. Statistical significance was determined by ANOVA with Newman–Keuls post hoc testing, unless otherwise noted.
Figure 2.
LM22A-4 reduces diffuse and aggregated huntingtin in nuclei of striatal and cortical neurons of R6/2 mice. A, Representative photomicrographs showing nuclear huntingtin immunostaining in striatum of R6/2 mice treated with vehicle (Veh; top) or LM22A-4 (LM-4; bottom). Diffuse huntingtin immunostaining was lighter and surrounded the huntingtin aggregates (arrows), which appeared smaller and less numerous in R6/2 mice given LM-4 (n = 9 mice) compared with those given Veh (n = 7 mice). Scale bar, 5 μm. Quantitative analyses of a sample field (0.063 mm2) in striatum (Str) and cortex (Cx) of one section per mouse confirmed these observations. B, The total area, number, and intensity of nuclei containing diffuse huntingtin staining decreased with LM-4 treatment in both brain areas (+p ≤ 0.05, ++p ≤ 0.01, and +++p ≤ 0.001 vs R6/2 veh). C, The total area occupied by huntingtin aggregates was reduced in Str and Cx due to a decrease in aggregate number and size (+p ≤ 0.05, ++p ≤ 0.01, and +++p ≤ 0.001 vs R6/2 veh). Results are normalized to the R6/2 vehicle group and expressed as mean ± SEM. Statistical significance was determined with a two-tailed Student's t test.
Figure 3.
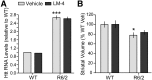
Huntingtin transcript levels and striatal atrophy are not affected by LM22A-4 in R6/2 mice. A, Data from qRT-PCR using primers recognizing both human and mouse huntingtin (htt) are shown (n = 2 for WTs and 3–4 R6/2 mice/group). Htt RNA levels were calculated and normalized to the internal control (GAPDH) and then expressed relative to the WT vehicle (Veh) group. Relative levels of htt transcript are increased in R6/2 striatum (***p = 0.001 vs WTs) and are not altered by LM22A-4 (LM-4). B, The volume of the striatum was significantly decreased in the Veh-treated R6/2 mice compared with the WTs (*p ≤ 0.05). LM-4 treatment did not prevent striatal atrophy in R6/2 mice (n = 9 mice/group). Results are expressed as mean ± SEM. Statistical significance was determined by ANOVA with Newman–Keuls post hoc testing.
Figure 4.
Decreases in striatal DARPP-32 levels are ameliorated by LM22A-4 in R6/2 mice. A, Representative DARPP-32 immunostaining in striatum of WT (left) and R6/2 mice (right) treated with vehicle (Veh; top) or LM22A-4 (LM-4; bottom) is shown. Scale bar, 80 μm. The immunostaining appeared lighter, especially in the neuropil, in R6/2 mice given Veh (n = 10) compared with the WT veh (n = 12), WT LM-4 (n = 10) and R6/2 LM-4 (n = 13) groups. B, Quantitative analyses showed that the decrease in area occupied by DARPP-32 immunostained cells and processes in Veh-treated R6/2 mice (*p ≤ 0.05 vs WT Veh) was prevented with LM-4 treatment (+p ≤ 0.05 vs R6/2 Veh). The area occupied by the immunostained cells and processes was expressed as a percentage of the total area analyzed and normalized by the WT Veh group. C, Representative Western blot of striatal homogenates from 11- to 12-week-old WT or R6/2 mice treated with Veh or LM-4 shows that DARPP-32 protein levels are decreased in the R6/2 Veh group compared with WTs (***p ≤ 0.001) and that LM-4 ameliorated this deficit (+p ≤ 0.05; two-tailed Student's t test). Immunobands from two mice per group are shown. For quantitation, samples from each mouse (n = 8–10 mice/group) were run in triplicate, values were normalized to the WT Veh group, and runs for each mouse were averaged. Results are expressed as mean ± SEM. Statistical significance was determined by ANOVA and Newman–Keuls post hoc test, unless otherwise noted.
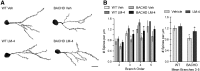
Figure 5.
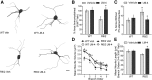
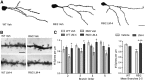
Degeneration of neurites from parvalbuminergic interneurons in R6/2 striatum is prevented by LM22A-4. A, Reconstructed drawings from Neurolucida tracings of PV-immunostained interneurons in striatum. Scale bar, 10 μm. B, Quantitative analyses showed that the decrease in area occupied by PV-immunostained neurons and neurites seen in vehicle (Veh)-treated R6/2 mice (*p ≤ 0.05 vs WT Veh) was prevented with LM22A-4 (LM-4) treatment (+p ≤ 0.05 vs R6/2 Veh; n = 9/group). The area occupied by the immunostained cells and processes was expressed as a percentage of the total area analyzed and normalized by the WT Veh group. C, R6/2 mice given Veh had a higher percentage of soma that lacked PV-stained neurites than WT mice (*p ≤ 0.05) and LM-4 prevented this deficit (+p ≤ 0.05 vs R6/2 Veh). D, E, PV-stained neurites of R6/2 vehicle mice also had reduced volumes on branch orders 1–4 (*p ≤ 0.05 vs WT Veh) and were shorter on branch orders 3–4, although this difference did not reach statistical significance. LM-4 increased the volume and length of the PV-stained neurites (+p ≤ 0.05 vs R6/2 Veh). Results are expressed as mean ± SEM. Statistical significance was determined by ANOVA with Newman–Keuls post hoc testing.
Figure 6.
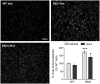
Inflammation is decreased by LM22A-4 in the R6/2 striatum. Immunostaining for IBA-1, a microglial marker, is shown in the striatum of a vehicle (Veh)-treated WT (top left) and R6/2 (top right) mouse, and an LM22A-4 (LM-4)-treated R6/2 mouse (bottom left). Scale bar, 80 μm. Quantitation showed that the area occupied by IBA-1 immunostaining increased significantly in Veh-treated R6/2 mice compared with WT mice (***p ≤ 0.001 vs WT Veh) and that LM-4 ameliorated this effect (+p ≤ 0.05; n = 9/group). The area occupied by the immunostained cells and processes was expressed as a percentage of the total area analyzed and normalized by the WT Veh group. Results are expressed as the mean ± SEM. Statistical significance was determined with an ANOVA and Newman–Keuls post hoc test.
Figure 7.
LM22A-4 prevents dendritic spine loss on R6/2 striatal MSNs. A, Reconstructed drawings from Neurolucida tracings of one dendritic tree of Golgi-stained MSNs in striatum. Scale bar, 10 μm. B, Spines on the fourth dendritic branch of MSNs from each of the four experimental groups are shown. Scale bar, 2.5 μm. C, Spine density on dendrites of MSNs was reduced in vehicle (Veh)-treated R6/2 mice (n = 7–9 mice/group; **p ≤ 0.01 vs WT Veh). This decrease was prevented by LM22A-4 (LM-4) on each branch order examined (*p ≤ 0.05 vs WT Veh and +p ≤ 0.05 vs R6/2 Veh; two-tailed Student's t tests; left graph) and this protective effect was particularly evident when data from branch orders 2–5 were combined (+++p < 0.001 vs R6/2 Veh; right graph). Results are expressed as mean ± SEM. Statistical significance was determined by ANOVA with Newman–Keuls post hoc testing.
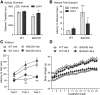
Figure 8.
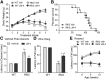
Effects of LM22A-4 on body weight, life span, and motor performance in R6/2 mice. A, Body weights of vehicle (Veh)-treated R6/2 mice were significantly less than WT mice given Veh or LM22A-4 (LM-4) starting at 7 weeks of age (fourth treatment week) and progressively decreased with aging. This decline was delayed by LM-4 treatment from the fifth to seventh treatment weeks (n = 35–37 mice/group).***p ≤ 0.001 vs WT Veh and +p ≤ 0.05 vs R6/2 Veh, two-way RM-ANOVA, with Bonferroni post-testing. B, Kaplan–Meier analysis curve showing that LM-4 does not affect survival of R6/2 mice (n = 10/group). C, Time to descend a vertical pole was longer for Veh-treated R6/2 mice than WTs (***p ≤ 0.001 vs WT Veh); LM-4 decreased descent time in R6/2 mice (++p ≤ 0.01 vs R6/2 Veh; n = 18/group). D, Hanging duration was shorter for R6/2 mice given Veh than WTs (***p ≤ 0.001 vs WT Veh); LM-4-treated R6/2 mice remained hanging longer than R6/2 Veh mice (+++p ≤ 0.001; n = 9–10/group). E, Rotarod performance was impaired in R6/2 mice (*p ≤ 0.05 and ***p ≤ 0.001 vs WT Veh) and was not affected by LM-4 treatment (n = 19–20/group). Results are expressed as mean ± SEM. Statistical significance was determined with an ANOVA and Newman–Keuls post hoc test, unless otherwise noted.
Figure 9.
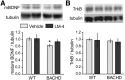
Mature BDNF levels are decreased while TrkB levels are unaltered in striatum of 8-month-old BACHD mice. Representative Western blots from BACHD mice (treated for 6 months beginning at 2 months of age) showing striatal protein levels of (A) mBDNF (∼14–15 kDa) and (B) TrkB. Immunobands from one mouse per group and corresponding densitometric analyses are shown. Corresponding tubulin immunobands from the stripped and reprobed blots are shown at the bottom of each gel panel. For quantitation, samples from each mouse (n = 5–9 mice/group) were run in duplicate, values were normalized to the WT vehicle group, and runs for each mouse were averaged. mBDNF protein levels are lower in striatum of vehicle-treated BACHD mice compared with WTs (*p ≤ 0.05). Striatal levels of TrkB were unaltered in BACHD mice. LM22A-4 (LM-4) did not significantly affect mBDNF or TrkB levels. Results are expressed as mean ± SEM, and, for comparison purposes, some nonadjacent lanes of Western blots were moved together and separated by spaces. Statistical significance was determined with an ANOVA with a Newman–Keuls post hoc test.
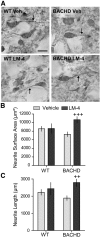
Figure 10.
LM22A-4 ameliorates degeneration of striatal DARPP-32 neurites in BACHD mice. A, DARPP-32 immunostaining in striatum of WT (left) and BACHD mice (right) treated with vehicle (Veh; top) or LM22A-4 (LM-4; bottom) is shown. Scale bar, 10 μm. The neurites of DARPP-32-stained neurons (A, arrows) of BACHD mice tend to have a smaller surface area (B) and shorter length (C) than those of WT mice (n = 8–9 mice/group; ANOVA p = 0.04, F = 3.24; p = 0.07, Student's t test WT Veh vs BACHD Veh). Treating BACHD mice with LM-4 significantly increased the surface area and length of the neurites containing DARPP-32 (++p = 0.005 and +++p = 0.001 vs BACHD veh, two-tailed Student's t tests).
Figure 11.
LM22A-4 prevents deficits in dendritic spines of BACHD striatal MSNs. A, Reconstructed drawings from Neurolucida tracings of one dendritic tree of Golgi-stained MSNs in striatum of WT (left) and BACHD mice (right) treated with vehicle (Veh; top) or LM22A-4 (LM-4; bottom). Scale bar, 10 μm. B, Spine density of MSNs was reduced in Veh-treated BACHD mice on dendritic branch orders 3–5 (*p ≤ 0.05 vs WT Veh, ANOVA and Newman–Keuls post hoc test, right graph) particularly on third and fourth orders (*p ≤ 0.05 vs WT Veh, Student's t test, left graph); this reduction was prevented by LM-4 (+p ≤ 0.04 vs BACHD Veh, Student's t test; n = 9–10/group). Results are expressed as mean ± SEM.
Figure 12.
Effects of LM22A-4 on motor performance and body weight in BACHD mice. A, Distance traveled during the first 10 min in an activity chamber was decreased in vehicle (Veh)-treated BACHD mice compared with WT mice (*p ≤ 0.05). This hypoactivity was prevented by LM22A-4 (LM-4) treatment (+p ≤ 0.05 vs BACHD Veh; n = 12–15/group). B, Downward climbing was also impaired in BACHD Veh mice (***p ≤ 0.001 vs WT Veh); LM-4 improved descent time of BACHD mice on the vertical pole test (+p ≤ 0.05 vs BACHD Veh; n = 15–22/group). C, Deficits in rotarod performance were seen at 5 (Test 2) and 8 (Test 3) months of age (both **p ≤ 0.01 vs WT Veh), but not at 2 months (Test 1); LM-4 had no effect on performance on this test (n = 15–22/group). D, Body weight was not affected by LM-4 treatment in WT or BACHD mice (n = 15–22/group). Results are expressed as mean ± SEM. Statistical significance was determined by ANOVA with Newman–Keuls post hoc testing.
Similar articles
- Imbalance of p75(NTR)/TrkB protein expression in Huntington's disease: implication for neuroprotective therapies.
Brito V, Puigdellívol M, Giralt A, del Toro D, Alberch J, Ginés S. Brito V, et al. Cell Death Dis. 2013 Apr 18;4(4):e595. doi: 10.1038/cddis.2013.116. Cell Death Dis. 2013. PMID: 23598407 Free PMC article. - Early Downregulation of p75NTR by Genetic and Pharmacological Approaches Delays the Onset of Motor Deficits and Striatal Dysfunction in Huntington's Disease Mice.
Suelves N, Miguez A, López-Benito S, Barriga GG, Giralt A, Alvarez-Periel E, Arévalo JC, Alberch J, Ginés S, Brito V. Suelves N, et al. Mol Neurobiol. 2019 Feb;56(2):935-953. doi: 10.1007/s12035-018-1126-5. Epub 2018 May 27. Mol Neurobiol. 2019. PMID: 29804232 - Brain-Derived Neurotrophic Factor Dysregulation as an Essential Pathological Feature in Huntington's Disease: Mechanisms and Potential Therapeutics.
Speidell A, Bin Abid N, Yano H. Speidell A, et al. Biomedicines. 2023 Aug 16;11(8):2275. doi: 10.3390/biomedicines11082275. Biomedicines. 2023. PMID: 37626771 Free PMC article. Review. - The neurotrophic receptor TrkB: a drug target in anti-cancer therapy?
Desmet CJ, Peeper DS. Desmet CJ, et al. Cell Mol Life Sci. 2006 Apr;63(7-8):755-9. doi: 10.1007/s00018-005-5490-8. Cell Mol Life Sci. 2006. PMID: 16568244 Free PMC article. Review.
Cited by
- TSPO-PET imaging using [18F]PBR06 is a potential translatable biomarker for treatment response in Huntington's disease: preclinical evidence with the p75NTR ligand LM11A-31.
Simmons DA, James ML, Belichenko NP, Semaan S, Condon C, Kuan J, Shuhendler AJ, Miao Z, Chin FT, Longo FM. Simmons DA, et al. Hum Mol Genet. 2018 Aug 15;27(16):2893-2912. doi: 10.1093/hmg/ddy202. Hum Mol Genet. 2018. PMID: 29860333 Free PMC article. - Fully human agonist antibodies to TrkB using autocrine cell-based selection from a combinatorial antibody library.
Merkouris S, Barde YA, Binley KE, Allen ND, Stepanov AV, Wu NC, Grande G, Lin CW, Li M, Nan X, Chacon-Fernandez P, DiStefano PS, Lindsay RM, Lerner RA, Xie J. Merkouris S, et al. Proc Natl Acad Sci U S A. 2018 Jul 24;115(30):E7023-E7032. doi: 10.1073/pnas.1806660115. Epub 2018 Jul 9. Proc Natl Acad Sci U S A. 2018. PMID: 29987039 Free PMC article. - Advances in developing novel therapeutic strategies for Alzheimer's disease.
Cao J, Hou J, Ping J, Cai D. Cao J, et al. Mol Neurodegener. 2018 Dec 12;13(1):64. doi: 10.1186/s13024-018-0299-8. Mol Neurodegener. 2018. PMID: 30541602 Free PMC article. Review. - Striatal Plasticity in L-DOPA- and Graft-Induced Dyskinesia; The Common Link?
Rylander Ottosson D, Lane E. Rylander Ottosson D, et al. Front Cell Neurosci. 2016 Feb 8;10:16. doi: 10.3389/fncel.2016.00016. eCollection 2016. Front Cell Neurosci. 2016. PMID: 26903804 Free PMC article. Review. - Neuroprotection by ADAM10 inhibition requires TrkB signaling in the Huntington's disease hippocampus.
Scolz A, Vezzoli E, Villa M, Talpo F, Cazzola J, Raffin F, Cordiglieri C, Falqui A, Pepe G, Maglione V, Besusso D, Biella G, Zuccato C. Scolz A, et al. Cell Mol Life Sci. 2024 Aug 7;81(1):333. doi: 10.1007/s00018-024-05382-1. Cell Mol Life Sci. 2024. PMID: 39112663 Free PMC article.
References
- Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, Cattaneo E, Marsh JL, Thompson LM. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS069375/NS/NINDS NIH HHS/United States
- R21 NS071024/NS/NINDS NIH HHS/United States
- R21 NS071024-01/NS/NINDS NIH HHS/United States
- P30 NS069375-01A1/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials