Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis - PubMed (original) (raw)
Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis
Jinju Wang et al. Oxid Med Cell Longev. 2013.
Abstract
Oxidative stress-induced endothelial dysfunction plays a key role in ischemia/reperfusion injury. Recent evidence indicates that endothelial progenitor cell-derived microvesicles (EPC-MVs) can promote angiogenesis of endothelial cells (ECs). Here, we investigated the potential effects of EPC-MVs on hypoxia/reoxygenation (H/R) injury in human brain microvascular ECs (hb-ECs). MVs were prepared from EPCs cultured in a serum deprivation (SD) medium (starving stress, sEPC-MVs) or SD medium containing tumor necrosis factor- α (TNFα) (apoptotic stress, aEPC-MVs). H/R injury model of hb-ECs was produced by 6 hr hypoxia (1% O2) and 24 hr reoxygenation. The H/R hb-ECs were co-cultured with EPC-MVs. Results showed that (1) H/R hb-ECs were dysfunctional and coupled with increased apoptosis and ROS overproduction; (2) under two different conditions, EPCs displayed remarkable difference in caspase 3 and miR126 expression, which were carried by the corresponsive EPC-MVs; (3) functionally, sEPC-MVs had beneficial effects on H/R hb-ECs, whereas aEPC-MVs had detrimental effects; (4) the diverse effects of sEPC-MVs and aEPC-MVs were associated with the changes in miR126 and eNOS expression and were abolished by PI3K inhibitor. In conclusion, sEPCs-MVs and aEPC-MVs are functionally different on hb-EC apoptosis and dysfunction via their carried RNAs associated with ROS production and PI3K/eNOS/NO pathway.
Figures
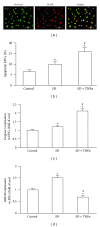
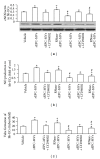
Figure 1
Effects of serum deprivation (SD) alone and SD plus TNF_α_ on EPC apoptosis, caspase 3, and miR126 expression. (a) Representative images showing EPC characterization of Bs-Lectin and Di-LDL double staining. Scale bar: 100 _μ_m. (b) Apoptosis (Annexin V+PI−) of stimulated EPCs. (c) Caspase 3 expression in stimulated EPCs. (d) MiR126 expression in stimulated EPCs. *P < 0.05, versus control; # P < 0.05, versus SD; N = 4/group.
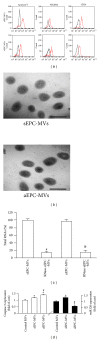
Figure 2
EPC-MV characterization, modification, caspase 3 and miR126 expression. (a) Flow cytometric plots showing Annexin V, CD34 and VEGFR2 expressions (isotype controls: left curves; antibodies: right curves) in EPC-MVs. (b) TEM image showing similar spherical morphology of sEPC-MVs and aEPC-MVs. Scale bar: 500 nm. (c) Summarized data showing effective digestion of EPC-MVs total RNAs by RNase treatment. (d) Caspase 3 and miR126 expression in control MVs (generated from basal condition), sEPC-MVs, and aEPC-MVs. *P < 0.05, versus control; # P < 0.05, versus sEPC-MVs; & P < 0.05, versus aEPC-MVs; N = 4/group. TEM and transmission electron microscopy.
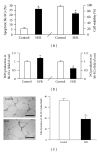
Figure 3
Effects of H/R on hb-EC viability and apoptosis, ROS and NO production, and tube formation. (a) Apoptosis (Annexin V+PI−) and cell viability. (b) ROS and NO production. (c) Tube formation ability. Scale bar: 200 _μ_m. *P < 0.05, versus control; N = 4/group.
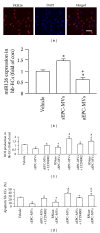
Figure 4
Effects of EPC-MVs on miR126 expression, ROS production, and apoptosis in H/R hb-ECs. (a) Representative images showing the merging of PKH26 labeled EPC-MVs with hb-ECs (red: PKH26; blue: DAPI). Scale bar: 100 _μ_m. (b) miR126 expression in H/R hb-ECs cocultured with aEPC-MVs or sEPC-MVs. (c) ROS production of H/R hb-ECs cocultured with aEPC-MVs or sEPC-MVs. (d) Apoptosis of H/R hb-ECs cocultured with aEPC-MVs or sEPC-MVs. *P < 0.05, versus vehicle; + P < 0.05, versus sEPC-MVs or aEPC-MVs; # P < 0.05, versus sEPC-MVs or RNase-sEPC-MVs; N = 4/group.
Figure 5
Effects of EPC-MVs on eNOS expression, NO production, and tube formation in H/R hb-ECs. (a) eNOS production of H/R hb-ECs cocultured with aEPC-MVs or sEPC-MVs. (b) NO production of H/R hb-ECs cocultured with aEPC-MVs or sEPC-MVs. (c) Tube formation ability of H/R hb-ECs cocultured with aEPC-MVs or sEPC-MVs. *P < 0.05, versus vehicle; + P < 0.05, versus sEPC-MVs or aEPC-MVs; # P < 0.05, versus sEPC-MVs or RNase-sEPC-MVs; N = 4/group.
Similar articles
- EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis.
Gu S, Zhang W, Chen J, Ma R, Xiao X, Ma X, Yao Z, Chen Y. Gu S, et al. PLoS One. 2014 Jan 2;9(1):e85396. doi: 10.1371/journal.pone.0085396. eCollection 2014. PLoS One. 2014. PMID: 24392165 Free PMC article. - ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway.
Zhang C, Wang J, Ma X, Wang W, Zhao B, Chen Y, Chen C, Bihl JC. Zhang C, et al. J Cell Mol Med. 2018 Mar;22(3):1873-1882. doi: 10.1111/jcmm.13471. Epub 2018 Jan 24. J Cell Mol Med. 2018. PMID: 29363860 Free PMC article. - Endothelial progenitor cells and neural progenitor cells synergistically protect cerebral endothelial cells from Hypoxia/reoxygenation-induced injury via activating the PI3K/Akt pathway.
Wang J, Chen Y, Yang Y, Xiao X, Chen S, Zhang C, Jacobs B, Zhao B, Bihl J, Chen Y. Wang J, et al. Mol Brain. 2016 Feb 3;9:12. doi: 10.1186/s13041-016-0193-7. Mol Brain. 2016. PMID: 26842559 Free PMC article. - Pathogenic roles of microvesicles in diabetic retinopathy.
Zhang W, Chen S, Liu ML. Zhang W, et al. Acta Pharmacol Sin. 2018 Jan;39(1):1-11. doi: 10.1038/aps.2017.77. Epub 2017 Jul 17. Acta Pharmacol Sin. 2018. PMID: 28713160 Free PMC article. Review. - Influence of statin use on endothelial function: from bench to clinics.
Martínez-González J, Badimon L. Martínez-González J, et al. Curr Pharm Des. 2007;13(17):1771-86. doi: 10.2174/138161207780831220. Curr Pharm Des. 2007. PMID: 17584107 Review.
Cited by
- Epigenetic Regulation of Oxidative Stress in Ischemic Stroke.
Zhao H, Han Z, Ji X, Luo Y. Zhao H, et al. Aging Dis. 2016 May 27;7(3):295-306. doi: 10.14336/AD.2015.1009. eCollection 2016 May. Aging Dis. 2016. PMID: 27330844 Free PMC article. Review. - Effects of Microvesicles on Cell Apoptosis under Hypoxia.
Guo Y, Tan J, Miao Y, Sun Z, Zhang Q. Guo Y, et al. Oxid Med Cell Longev. 2019 Apr 17;2019:5972152. doi: 10.1155/2019/5972152. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31178970 Free PMC article. Review. - Microvesicles: ROS scavengers and ROS producers.
Bodega G, Alique M, Puebla L, Carracedo J, Ramírez RM. Bodega G, et al. J Extracell Vesicles. 2019 Jun 17;8(1):1626654. doi: 10.1080/20013078.2019.1626654. eCollection 2019. J Extracell Vesicles. 2019. PMID: 31258880 Free PMC article. Review. - The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway.
Wu K, Yang Y, Zhong Y, Ammar HM, Zhang P, Guo R, Liu H, Cheng C, Koroscil TM, Chen Y, Liu S, Bihl JC. Wu K, et al. Am J Physiol Endocrinol Metab. 2016 May 15;310(10):E828-37. doi: 10.1152/ajpendo.00056.2016. Epub 2016 Mar 8. Am J Physiol Endocrinol Metab. 2016. PMID: 26956185 Free PMC article. - Picroside II protects the blood-brain barrier by inhibiting the oxidative signaling pathway in cerebral ischemia-reperfusion injury.
Zhai L, Liu M, Wang T, Zhang H, Li S, Guo Y. Zhai L, et al. PLoS One. 2017 Apr 7;12(4):e0174414. doi: 10.1371/journal.pone.0174414. eCollection 2017. PLoS One. 2017. PMID: 28388666 Free PMC article.
References
- Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radical Biology and Medicine. 2005;39(4):429–443. - PubMed
- Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovascular Research. 2008;78(3):413–421. - PubMed
- Charwat S, Gyöngyösi M, Lang I, et al. Role of adult bone marrow stem cells in the repair of ischemic myocardium: current state of the art. Experimental Hematology. 2008;36(6):672–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials