Advanced glycation end products (AGE) and diabetes: cause, effect, or both? - PubMed (original) (raw)
Review
Advanced glycation end products (AGE) and diabetes: cause, effect, or both?
Helen Vlassara et al. Curr Diab Rep. 2014 Jan.
Abstract
Despite new and effective drug therapies, insulin resistance (IR), type 2 diabetes mellitus (T2D) and its complications remain major medical challenges. It is accepted that IR, often associated with over-nutrition and obesity, results from chronically elevated oxidant stress (OS) and chronic inflammation. Less acknowledged is that a major cause for this inflammation is excessive consumption of advanced glycation end products (AGEs) with the standard western diet. AGEs, which were largely thought as oxidative derivatives resulting from diabetic hyperglycemia, are increasingly seen as a potential risk for islet β-cell injury, peripheral IR and diabetes. Here we discuss the relationships between exogenous AGEs, chronic inflammation, IR, and T2D. We propose that under chronic exogenous oxidant AGE pressure the depletion of innate defense mechanisms is an important factor, which raises susceptibility to inflammation, IR, T2D and its complications. Finally we review evidence on dietary AGE restriction as a nonpharmacologic intervention, which effectively lowers AGEs, restores innate defenses and improves IR, thus, offering new perspectives on diabetes etiology and therapy.
Conflict of interest statement
Conflict of Interest
Helen Vlassara has received grant support and support for travel to meetings for the study or otherwise from Sanofi for a co-investigator-initiated clinical investigation. She has patents (planned, pending or issued) from Cell Biolabs for development of monoclonal antibody, and receives royalties for monoclonal antibody. Her husband is principal investigator in investigator-initiated clinical trial, supported by Sanofi.
Jaime Uribarri is a co-author on a book on AGE-less Diet.
Figures
Figure 1
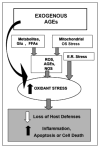
Native and exogenous sources of oxidants, a most notable one being food derived AGEs, contribute to cellular and tissue injury in chronic diabetes.
Figure 2
AGE restriction protects islet structure and function in mice genetically predisposed to type 1 (A) and type 2 diabetes (B). Non-obese diabetic (NOD) mice (A), and db/db +/+ mice (B) were exposed to either standard or Low-AGE diet for life. Pancreatic islets from mice on AGE-restriction did not display the mononuclear cell infiltration, typical of NOD mice fed a standard diet (AGE-rich) (H&E mag.x400,). Similarly, islets from age-matched d_b/db+/+_ mice, after AGE-restriction, showed intact insulin production compared to those on regular diet. (insulin staining, mag.x400).
Figure 3
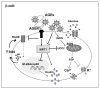
Dietary AGEs can impair insulin secretion in pancreatic islet β-cells. Pro-oxidant AGEs can induce iNOS, and the generation of mitochondrial ROS, suppressing cytochrome-C levels and ATP generation, reducing insulin release. They can also suppress the deacetylase SIRT1, which regulates UCP2, thus impairing membrane depolarization and β-cell secretory function.
Figure 4
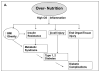
Traditional and non-traditional pathways to T1/T2D and complications. A, Over-nutrition promotes obesity and insulin resistance as well as increased OS, inflammation and β-cell injury leading to diabetes by unclear mechanisms. B, Modern diet, typically rich in animal food products, is also generally subject to heat-exposure. The resulting excess of oxidant AGEs include flavorful appetite-enhancing substances, which simultaneously promote food over-consumption and obesity, as well as insulin resistance, beta-cell injury and diabetes (type 1 or 2).
Figure 4
Traditional and non-traditional pathways to T1/T2D and complications. A, Over-nutrition promotes obesity and insulin resistance as well as increased OS, inflammation and β-cell injury leading to diabetes by unclear mechanisms. B, Modern diet, typically rich in animal food products, is also generally subject to heat-exposure. The resulting excess of oxidant AGEs include flavorful appetite-enhancing substances, which simultaneously promote food over-consumption and obesity, as well as insulin resistance, beta-cell injury and diabetes (type 1 or 2).
Figure 5
Partial list of known properties of AGER1, the best characterized anti-AGE receptor involved in the removal of AGEs, as well as in the maintenance of host defenses controlling their pro-inflammatory effects. Loss of function of AGER1 in chronic diabetes and other conditions of sustained oxidant AGE pressure is thought as one way by which depleted host defenses and anti-oxidant mechanisms can lead to the maladaptive inflammatory responses underlying diabetes and its complications.
Similar articles
- Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1.
Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Cai W, et al. Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):15888-93. doi: 10.1073/pnas.1205847109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908267 Free PMC article. - Cereal polyphenols inhibition mechanisms on advanced glycation end products and regulation on type 2 diabetes.
Dong L, Li Y, Chen Q, Liu Y, Wu Z, Pan D, Yan N, Liu L. Dong L, et al. Crit Rev Food Sci Nutr. 2024 Sep;64(26):9495-9513. doi: 10.1080/10408398.2023.2213768. Epub 2023 May 24. Crit Rev Food Sci Nutr. 2024. PMID: 37222572 Review. - Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1.
Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H. Uribarri J, et al. Diabetes Care. 2011 Jul;34(7):1610-6. doi: 10.2337/dc11-0091. Diabetes Care. 2011. PMID: 21709297 Free PMC article. Clinical Trial. - The Role of Dietary Advanced Glycation End Products in Metabolic Dysfunction.
Sergi D, Boulestin H, Campbell FM, Williams LM. Sergi D, et al. Mol Nutr Food Res. 2021 Jan;65(1):e1900934. doi: 10.1002/mnfr.201900934. Epub 2020 Apr 20. Mol Nutr Food Res. 2021. PMID: 32246887 Review. - AGE restriction in diabetes mellitus: a paradigm shift.
Vlassara H, Striker GE. Vlassara H, et al. Nat Rev Endocrinol. 2011 May 24;7(9):526-39. doi: 10.1038/nrendo.2011.74. Nat Rev Endocrinol. 2011. PMID: 21610689 Free PMC article. Review.
Cited by
- The AGE-RAGE Axis and Its Relationship to Markers of Cardiovascular Disease in Newly Diagnosed Diabetic Patients.
Villegas-Rodríguez ME, Uribarri J, Solorio-Meza SE, Fajardo-Araujo ME, Cai W, Torres-Graciano S, Rangel-Salazar R, Wrobel K, Garay-Sevilla ME. Villegas-Rodríguez ME, et al. PLoS One. 2016 Jul 19;11(7):e0159175. doi: 10.1371/journal.pone.0159175. eCollection 2016. PLoS One. 2016. PMID: 27434539 Free PMC article. - Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic rats.
Govindaraj J, Sorimuthu Pillai S. Govindaraj J, et al. Mol Cell Biochem. 2015 Jun;404(1-2):143-59. doi: 10.1007/s11010-015-2374-6. Epub 2015 Mar 4. Mol Cell Biochem. 2015. PMID: 25735949 - Glycemic management is inversely related to skeletal muscle microvascular endothelial function in patients with type 2 diabetes.
Bock JM, Hughes WE, Ueda K, Feider AJ, Hanada S, Casey DP. Bock JM, et al. Physiol Rep. 2021 Mar;9(5):e14764. doi: 10.14814/phy2.14764. Physiol Rep. 2021. PMID: 33660935 Free PMC article. - Mineral and cross-linking in collagen fibrils: The mechanical behavior of bone tissue at the nano-scale.
Kamml J, Acevedo C, Kammer DS. Kamml J, et al. J Mech Behav Biomed Mater. 2024 Nov;159:106697. doi: 10.1016/j.jmbbm.2024.106697. Epub 2024 Aug 22. J Mech Behav Biomed Mater. 2024. PMID: 39182252 Free PMC article. - Sulforaphane Inhibits MGO-AGE-Mediated Neuroinflammation by Suppressing NF-κB, MAPK, and AGE-RAGE Signaling Pathways in Microglial Cells.
Subedi L, Lee JH, Gaire BP, Kim SY. Subedi L, et al. Antioxidants (Basel). 2020 Aug 26;9(9):792. doi: 10.3390/antiox9090792. Antioxidants (Basel). 2020. PMID: 32859007 Free PMC article.
References
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14 (Suppl 5):S1–85. - PubMed
- Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29:1420–32. - PubMed
- Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. Adults Diabetes Care. 2004;27:2444–9. - PubMed
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. No authors listed. - PubMed
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. No authors listed. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG-23188/AG/NIA NIH HHS/United States
- R01 AG009453/AG/NIA NIH HHS/United States
- R01 DK091231/DK/NIDDK NIH HHS/United States
- M01 RR000071/RR/NCRR NIH HHS/United States
- M01-RR-00071/RR/NCRR NIH HHS/United States
- AG-09453/AG/NIA NIH HHS/United States
- R37 AG023188/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical