Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections - PubMed (original) (raw)
Review
Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections
Pierre Cornelis et al. Front Cell Infect Microbiol. 2013.
Abstract
Pseudomonas aeruginosa is a Gram-negative γ-Proteobacterium which is known for its capacity to colonize various niches, including some invertebrate and vertebrate hosts, making it one of the most frequent bacteria causing opportunistic infections. P. aeruginosa is able to cause acute as well as chronic infections and it uses different colonization and virulence factors to do so. Infections range from septicemia, urinary infections, burn wound colonization, and chronic colonization of the lungs of cystic fibrosis patients. Like the vast majority of organisms, P. aeruginosa needs iron to sustain growth. P. aeruginosa utilizes different strategies to take up iron, depending on the type of infection it causes. Two siderophores are produced by this bacterium, pyoverdine and pyochelin, characterized by high and low affinities for iron respectively. P. aeruginosa is also able to utilize different siderophores from other microorganisms (siderophore piracy). It can also take up heme from hemoproteins via two different systems. Under microaerobic or anaerobic conditions, P. aeruginosa is also able to take up ferrous iron via its Feo system using redox-cycling phenazines. Depending on the type of infection, P. aeruginosa can therefore adapt by switching from one iron uptake system to another as we will describe in this short review.
Keywords: Feo; Pseudomonas aeruginosa; heme uptake; iron; phenazines; pyochelin; pyoverdine; siderophores.
Figures
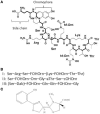
Figure 1
(A) Structure of the P. aeruginosa type I pyoverdine [taken from Ravel and Cornelis (2003)]. (B) Sequence of the peptide chains from P. aeruginosa type I, II, and III pyoverdines. (C) Structure of the second P. aeruginosa siderophore pyochelin.
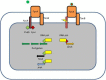
Figure 2
Ferripyoverdine is also a signal molecule. The left panel shows the FpvA receptor empty since only apo-pyoverdine is present. FpvA is a TonB-dependent receptor which is also associated with the FpvR anti-σ factor. FpvR sequesters two ECF σ, PvdS and FpvI via its cytoplasmic domain. In this condition there is little possibility for the respective σ factors to associate with the core RNA polymerase and the pyoverdine biosynthesis genes are not transcribed (PvdS) neither the fpvA gene (FpvI). The right panel shows what happens when ferripyoverdine binds to the FpvA receptor. This binding triggers a conformational change resulting in the proteolysis of FpvR, which liberates the two σ factors which now can associate with the RNA polymerase. PvdS is not only involved in the transcription of pyoverdine genes, but also in the expression of two virulence genes, prpL encoding an extracellular protease and exoA encoding the potent exotoxin A.
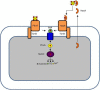
Figure 3
P. aeruginosa has two heme uptake systems, Phu, and Has. The PhuR TonB-dependent receptor binds directly hemoproteins extracting heme while the HasR receptor binds heme complexed to a secreted hemophore protein HasA. In the periplasm heme is bound to a periplasmic protein which delivers it to an ABC transporter. In the cytoplasm heme is directed to the heme oxygenase HemO by the PhuS chaperone. HemO cleaves the tetrapyrrole ring, leaving biliverdin, CO, and Fe2+.
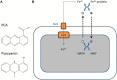
Figure 4
(A) Structure of the two major P. aeruginosa phenazines, phenazine-1-carboxylic acid (PCA), and pyocyanin. (B) Reduced PCA (white-filled circle) is excreted out of the cell and is oxidized (red-filled circle) resulting in the reduction of Fe3+ to Fe2+. The oxidized phenazine is recycled inside the cell where it is reduced again with simultaneous oxidation of NADH to NAD (Wang et al., 2011).
Similar articles
- In vitro lung epithelial cell model reveals novel roles for Pseudomonas aeruginosa siderophores.
Kang D, Xu Q, Kirienko NV. Kang D, et al. Microbiol Spectr. 2024 Mar 5;12(3):e0369323. doi: 10.1128/spectrum.03693-23. Epub 2024 Feb 5. Microbiol Spectr. 2024. PMID: 38311809 Free PMC article. - Phenotypic Adaption of Pseudomonas aeruginosa by Hacking Siderophores Produced by Other Microorganisms.
Perraud Q, Cantero P, Roche B, Gasser V, Normant VP, Kuhn L, Hammann P, Mislin GLA, Ehret-Sabatier L, Schalk IJ. Perraud Q, et al. Mol Cell Proteomics. 2020 Apr;19(4):589-607. doi: 10.1074/mcp.RA119.001829. Epub 2020 Feb 5. Mol Cell Proteomics. 2020. PMID: 32024770 Free PMC article. - Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection.
Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, Facchini M, Pasquali P, Bragonzi A, Visca P. Minandri F, et al. Infect Immun. 2016 Jul 21;84(8):2324-2335. doi: 10.1128/IAI.00098-16. Print 2016 Aug. Infect Immun. 2016. PMID: 27271740 Free PMC article. - High affinity iron uptake by pyoverdine in Pseudomonas aeruginosa involves multiple regulators besides Fur, PvdS, and FpvI.
Cornelis P, Tahrioui A, Lesouhaitier O, Bouffartigues E, Feuilloley M, Baysse C, Chevalier S. Cornelis P, et al. Biometals. 2023 Apr;36(2):255-261. doi: 10.1007/s10534-022-00369-6. Epub 2022 Feb 16. Biometals. 2023. PMID: 35171432 Review. - Pseudomonas aeruginosa virulence attenuation by inhibiting siderophore functions.
Jeong GJ, Khan F, Khan S, Tabassum N, Mehta S, Kim YM. Jeong GJ, et al. Appl Microbiol Biotechnol. 2023 Feb;107(4):1019-1038. doi: 10.1007/s00253-022-12347-6. Epub 2023 Jan 12. Appl Microbiol Biotechnol. 2023. PMID: 36633626 Review.
Cited by
- Activation of CzcS/CzcR during zinc excess regulates copper tolerance and pyochelin biosynthesis of Pseudomonas aeruginosa.
Li T, Cao H, Duan C, Chen S, Xu Z. Li T, et al. Appl Environ Microbiol. 2024 Mar 20;90(3):e0232723. doi: 10.1128/aem.02327-23. Epub 2024 Feb 20. Appl Environ Microbiol. 2024. PMID: 38376236 Free PMC article. - During bacteremia, Pseudomonas aeruginosa PAO1 adapts by altering the expression of numerous virulence genes including those involved in quorum sensing.
Beasley KL, Cristy SA, Elmassry MM, Dzvova N, Colmer-Hamood JA, Hamood AN. Beasley KL, et al. PLoS One. 2020 Oct 15;15(10):e0240351. doi: 10.1371/journal.pone.0240351. eCollection 2020. PLoS One. 2020. PMID: 33057423 Free PMC article. - Synthesis and evaluation of an amphiphilic deferoxamine:gallium-conjugated cationic random copolymer against a murine wound healing infection model of Pseudomonas aeruginosa.
Qiao J, Liu Z, Cui S, Nagy T, Xiong MP. Qiao J, et al. Acta Biomater. 2021 May;126:384-393. doi: 10.1016/j.actbio.2021.03.005. Epub 2021 Mar 8. Acta Biomater. 2021. PMID: 33705987 Free PMC article. - In vitro lung epithelial cell model reveals novel roles for Pseudomonas aeruginosa siderophores.
Kang D, Xu Q, Kirienko NV. Kang D, et al. Microbiol Spectr. 2024 Mar 5;12(3):e0369323. doi: 10.1128/spectrum.03693-23. Epub 2024 Feb 5. Microbiol Spectr. 2024. PMID: 38311809 Free PMC article. - Editorial: Role of Iron in Bacterial Pathogenesis.
Zughaier SM, Cornelis P. Zughaier SM, et al. Front Cell Infect Microbiol. 2018 Oct 16;8:344. doi: 10.3389/fcimb.2018.00344. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30460202 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical